Meerwein, H., Büchner, E. & van Emster, K. Über die Einwirkung aromatischer Diazoverbindungen auf α,β-ungesättigte Carbonylverbindungen. J. Prakt. Chem. 152, 237–266 (1939).
Hari, D. P. & König, B. The photocatalyzed meerwein arylation: Classic reaction of aryl diazonium salts in a new light. Angew. Chem. Int. Ed. 52, 4734–4743 (2013).
Ghosh, I., Marzo, L., Das, A., Shaikh, R. & König, B. Visible light mediated photoredox catalytic arylation reactions. Acc. Chem. Res. 49, 1566–1577 (2016).
Kvasovs, N. & Gevorgyan, V. Contemporary methods for generation of aryl radicals. Chem. Soc. Rev. 50, 2244–2259 (2021).
Galli, C. Radical reactions of arenediazonium ions: An easy entry into the chemistry of the aryl radical. Chem. Rev. 88, 765–792 (1988).
Liu, W., Yang, X., Gao, Y. & Li, C.-J. Simple and efficient generation of aryl radicals from aryl triflates: Synthesis of aryl boronates and aryl iodides at room temperature. J. Am. Chem. Soc. 139, 8621–8627 (2017).
Hu, C.-H. et al. Organic charge-transfer complex induces chemoselective decarboxylation to aryl radicals for general functionalization. Chem 9, 2997–3012 (2023).
Ji, C.-L. et al. Photoinduced activation of alkyl chlorides. Chem. Soc. Rev. 52, 6120–6138 (2023).
Chen, J.-R., Hu, X.-Q., Lu, L.-Q. & Xiao, W.-J. Visible light photoredox-controlled reactions of N-radicals and radical ions. Chem. Soc. Rev. 45, 2044–2056 (2016).
Xuan, J., He, X.-K. & Xiao, W.-J. Visible light-promoted ring-opening functionalization of three-membered carbo- and heterocycles. Chem. Soc. Rev. 49, 2546–2556 (2020).
Luo, M.-J., Xiao, Q. & Li, J.-H. Electro-/photocatalytic alkene-derived radical cation chemistry: recent advances in synthetic applications. Chem. Soc. Rev. 51, 7206–7237 (2022).
Twilton, J. et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 1, 0052 (2017).
Skubi, K. L., Blum, T. R. & Yoon, T. P. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 116, 10035–10074 (2016).
Ang, H. T., Miao, Y., Ravelli, D. & Wu, J. Pyridine N-oxides as hydrogen atom transfer reagents for site-selective photoinduced C(sp3)–H functionalization. Nat. Synth. 3, 568–575 (2024).
Lu, L.-Q., Li, T.-R., Wang, Q. & Xiao, W.-J. Beyond sulfide-centric catalysis: Recent advances in the catalytic cyclization reactions of sulfur ylides. Chem. Soc. Rev. 46, 4135–4149 (2017).
Juliá, F., Constantin, T. & Leonori, D. Applications of Halogen-atom transfer (XAT) for the generation of carbon radicals in synthetic photochemistry and photocatalysis. Chem. Rev. 122, 2292–2352 (2022).
Yi, H. et al. Recent advances in radical C–H activation/radical cross-coupling. Chem. Rev. 117, 9016–9085 (2017).
Chen, C., Wang, M., Lu, H., Zhao, B. & Shi, Z. Enabling the use of alkyl thianthrenium salts in cross-coupling reactions by copper catalysis. Angew. Chem. Int. Ed. 60, 21756–21760 (2021).
Zeng, L. et al. Iodoarene-directed photoredox β-C(sp3)–H arylation of 1-(o-iodoaryl)alkan-1-ones with cyanoarenes via halogen atom transfer and hydrogen atom transfer. Chem. Sci. 15, 6522–6529 (2024).
Chen, X.-Y. et al. Cu-mediated thianthrenation and phenoxathiination of arylborons. J. Am. Chem. Soc. 145, 10431–10440 (2023).
Alvarez, E. M., Karl, T., Berger, F., Torkowski, L. & Ritter, T. Late-stage heteroarylation of hetero(aryl)sulfonium salts activated by α-amino alkyl radicals. Angew. Chem. Int. Ed. 60, 13609–13613 (2021).
Meng, H., Liu, M.-S. & Shu, W. Organothianthrenium salts: synthesis and utilization. Chem. Sci. 13, 13690–13707 (2022).
Berger, F. et al. Site-selective and versatile aromatic C−H functionalization by thianthrenation. Nature 567, 223–228 (2019).
Tang, S., Zhao, X., Yang, L., Li, B. & Wang, B. Copper-catalyzed carboxylation of aryl thianthrenium salts with CO2. Angew. Chem. Int. Ed. 61, e202212975 (2022).
Ni, S. et al. C–heteroatom coupling with electron-rich aryls enabled by nickel catalysis and light. Nat. Catal. 7, 733–741 (2024).
Dewanji, A. et al. A general arene C–H functionalization strategy via electron donor–acceptor complex photoactivation. Nat. Chem. 15, 43–52 (2023).
Cabrera-Afonso, M. J., Granados, A. & Molander, G. A. Sustainable thioetherification via electron donor–acceptor photoactivation using thianthrenium salts. Angew. Chem. Int. Ed. 61, e202202706 (2022).
van Dalsen, L., Brown, R. E., Rossi-Ashton, J. A. & Procter, D. J. Sulfonium salts as acceptors in electron donor-acceptor complexes. Angew. Chem. Int. Ed. 62, e202303104 (2023).
Li, B. et al. Catalyst-free C(sp2)-H borylation through aryl radical generation from thiophenium salts via electron donor–acceptor complex formation. Org. Lett. 24, 7434–7439 (2022).
Gallego-Gamo, A. et al. Site-selective and metal-free C–H phosphonation of arenes via photoactivation of thianthrenium salts. RSC Adv. 13, 23359–23364 (2023).
Zhang, Y.-L., Wang, G.-H., Wu, Y., Zhu, C.-Y. & Wang, P. Construction of α-amino azines via thianthrenation-enabled photocatalyzed hydroarylation of azine-substituted enamides with arenes. Org. Lett. 23, 8522–8526 (2021).
Wu, J. et al. Para-selective borylation of monosubstituted benzenes using a transient mediator. Sci. China Chem. 63, 336–340 (2020).
Sun, K. et al. A general electron donor–acceptor complex for photoactivation of arenes via thianthrenation. Chem. Sci. 13, 5659–5666 (2022).
Zhang, Y. et al. Radical C–H sulfonation of arenes: its applications on bioactive and DNA-encoded molecules. Org. Lett. 24, 7961–7966 (2022).
Zhao, Y., Empel, C., Liang, W., Koenigs, R. M. & Patureau, F. W. Gem-difluoroallylation of aryl sulfonium salts. Org. Lett. 24, 8753–8758 (2022).
Yang, K., Feng, T. & Qiu, Y. Organo-Mediator Enabled Electrochemical Deuteration of Styrenes. Angew. Chem. Int. Ed. 62, e202312803 (2023).
Loh, Y. Y. et al. Photoredox-catalyzed deuteration and tritiation of pharmaceutical compounds. Science 358, 1182–1187 (2017).
Li, W. et al. Scalable and selective deuteration of (hetero)arenes. Nat. Chem. 14, 334–341 (2022).
Li, N. et al. Highly selective single and multiple deuteration of unactivated C(sp3)-H bonds. Nat. Commun. 13, 4224 (2022).
Lin, Z.-H., Yao, Y.-F. & Zhang, C.-P. Deuteration of arylthianthren-5-ium salts in CD3OD. Org. Lett. 24, 8417–8422 (2022).
Zhang, M., Yuan, X.-A., Zhu, C. & Xie, J. Deoxygenative deuteration of carboxylic acids with D2O. Angew. Chem. Int. Ed. 58, 312–316 (2019).
Zhao, D., Petzold, R., Yan, J., Muri, D. & Ritter, T. Tritiation of aryl thianthrenium salts with a molecular palladium catalyst. Nature 600, 444–449 (2021).
Lang, Y., Peng, X., Li, C.-J. & Zeng, H. Photoinduced catalyst-free deborylation–deuteration of arylboronic acids with D2O. Green. Chem. 22, 6323–6327 (2020).
Li, P. et al. Facile and general electrochemical deuteration of unactivated alkyl halides. Nat. Commun. 13, 3774 (2022).
Zhao, Z. et al. Electrochemical C−H deuteration of pyridine derivatives with D2O. Nat. Commun. 15, 3832 (2024).
Li, N., Li, Y., Wu, X., Zhu, C. & Xie, J. Radical deuteration. Chem. Soc. Rev. 51, 6291–6306 (2022).
Ou, W., Qiu, C. & Su, C. Photo- and electro-catalytic deuteration of feedstock chemicals and pharmaceuticals: A review. Chin. J. Catal. 43, 956–970 (2021).
Zhou, R., Ma, L., Yang, X. & Cao, J. Recent advances in visible-light photocatalytic deuteration reactions. Org. Chem. Front. 8, 426–444 (2021).
Ho, J. et al. Chloroform as a hydrogen atom donor in barton reductive decarboxylation reactions. J. Org. Chem. 78, 6677–6687 (2013).
Davico, G. E., Bierbaum, V. M., DePuy, C. H., Ellison, G. B. & Squires, R. R. The C-H bond energy of benzene. J. Am. Chem. Soc. 117, 2590–2599 (1995).
Patra, T., Mukherjee, S., Ma, J., Strieth-Kalthoff, F. & Glorius, F. Visible-light-photosensitized aryl and alkyl decarboxylative functionalization reactions. Angew. Chem. Int. Ed. 58, 10514–10520 (2019).
Tilby, M. J. et al. Photocatalytic late-stage functionalization of sulfonamides via sulfonyl radical intermediates. ACS Catal. 12, 6060–6067 (2022).
Majhi, J. et al. Metal-free photochemical imino-alkylation of alkenes with bifunctional oxime esters. J. Am. Chem. Soc. 144, 15871–15878 (2022).
Qin, J.-H., Luo, M.-J., An, D.-L. & Li, J.-H. Electrochemical 1,2-diarylation of alkenes enabled by direct dual c–h functionalizations of electron-rich aromatic hydrocarbons. Angew. Chem. Int. Ed. 60, 1861–1868 (2021).
Min, X.-T. et al. Rhodium-catalyzed deuterated tsuji–wilkinson decarbonylation of aldehydes with deuterium oxide. J. Am. Chem. Soc. 144, 11081–11087 (2022).
Shi, Q. et al. Visible-light mediated catalytic asymmetric radical deuteration at non-benzylic positions. Nat. Commun. 13, 4453 (2022).
Luo, J., Lu, L., Montag, M., Liang, Y. & Milstein, D. Hydrogenative alkene perdeuteration aided by a transient cooperative ligand. Nat. Chem. 15, 1384–1390 (2023).
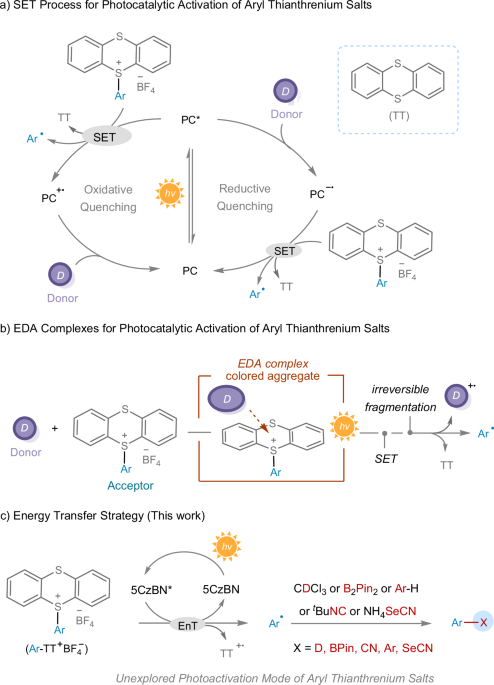
Strieth-Kalthoff, F., James, M. J., Teders, M., Pitzer, L. & Glorius, F. Energy transfer catalysis mediated by visible light: principles, applications, directions. Chem. Soc. Rev. 47, 7190–7202 (2018).
Soni, V. K. et al. Reactivity tuning for radical–radical cross-coupling via selective photocatalytic energy transfer: Access to amine building blocks. ACS Catal. 9, 10454–10463 (2019).
De Sorgo, M., Wasserman, B. & Szwarc, M. Aggregation of salts of thianthrene radical cations. J. Phys. Chem. 76, 3468–3471 (1972).
Shine, H. J. & Murata, Y. Kinetics and mechanism of the reaction of the thianthrene cation radical with water. J. Am. Chem. Soc. 91, 1872–1874 (1969).