We used unfixed human excised abdominal skin tissue obtained from BIOPREDIC International (Rennes, France) to verify the impact of the culture method with and without microneedles (as described later). For live imaging purposes, excised abdominal skin tissue was provided by the University School of Medicine. The study protocol adhered to the ethical guidelines of the University’s Ethics Committee (approval number: B20-333) and the Ethics Committee of the FANCL Co., Ltd. (approval number: C2020-039). The research was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from each patient before study enrollment.
Culture of human skin tissue for evaluation of culture methods
Each skin tissue excised from the human abdomen was transported at 4 °C to the laboratory within 24 h. After arrival, unfixed human skin tissues were immediately cut into 9 mm-diameter and 900 μm-thick pieces and used to start a culture employing two different methods. For the conventional osmosis culture (control), each skin piece was placed in a cell culture insert (Thermo Fisher Scientific, Waltham, MA, USA), allowing the medium to permeate from the bottom through a polycarbonate membrane. In contrast, for the infusion culture, in addition to the above methods, a microneedle was inserted into a piece of skin through the epidermal surface, and a medium was injected into the skin. In the former method (control), 4 mL of Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM F-12) (Thermo Fisher Scientific) was placed in the 6-well plate (Sumiron Co., Ltd., Osaka, JAPAN), which is a cell insert tray. For the latter, 2 mL of DMEM F-12 was placed in the 6-well plate, and 2 mL of DMEM F-12 was injected into the skin tissue via the microneedle. The cultures were maintained at 37 °C and 5% CO2. The culture medium was collected and replaced on days 1, 2, 5, 7, 9, and 11. Each skin sample was fixed in 4% paraformaldehyde in PBS before or after 5 and 11 d of culture. This culture method was based on the protocol for the NativeSkin® (Genoskin, Toulouse, France) model. Still, the added volume of the medium was slightly greater than in the original protocol, considering the frequency of the replacement of the medium.
Microneedle
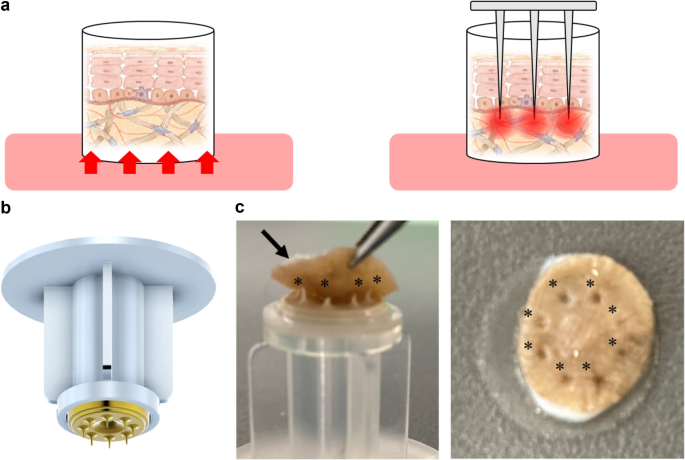
The microneedles used for the culture were provided by Think-Lands Co., Ltd. (Kawasaki, Japan, https://www.think-lands.co.jp/). To ensure compatibility with the strength and thickness of the excised skin tissue and to prevent needle clogging, we developed a disk-shaped component featuring eight microneedles with diameters of 0.1 mm and lengths of 1.0–1.2 mm (Fig. 4). This needle component was attached to the tip of an infusion tube and inserted from the epidermal side of the skin, allowing fluid infusion into the skin tissue via the eight needles. The infusion rate was regulated using a syringe pump (AS ONE CORPORATION, Osaka, Japan).
Schematic diagrams of each culture method and actual images of microneedles. (a) In the traditional culture method, skin tissue is placed in a cell insert, and the culture medium infiltrates from the dermal side through a polycarbonate membrane (left). In the culture method using microneedles, several needles with a diameter of 0.1 mm and a length of 1.0–1.2 mm are inserted into the dermis of the skin tissue (right). (b) A photograph of microneedles. An attachment fitted with eight microneedles. (c) Photograph fitting to the excised skin tissue (left). Eight microneedles (black asterisk) were inserted into the excised skin tissue (black arrow). Photo after insertion (right). Photograph taken from the surface of the skin (right). A black asterisk indicates the location of the inserted trace.
Immunofluorescence staining and TUNEL staining
Each fixed skin tissue sample was sliced into 1 mm-thick sections oriented along the epidermis–dermis axis. The sections were washed with 0.05% Tween 20 in PBS and blocked with Starting Block blocking buffer (Thermo Fisher Scientific). They were then labeled with anti-tropoelastin mouse monoclonal antibody (Santa Cruz Biotechnology, Dallas, TX, USA) and anti-COL VII rabbit polyclonal antibody (Abcam, Cambridge, MA, USA), both diluted to 1:500 in the Starting Block blocking buffer. After washing, the sections were stained with Goat Anti-Mouse IgG H&L Alexa Fluor® 488 and Goat Anti-Rabbit IgG H&L Alexa Fluor® 647 (Thermo Fisher Scientific) at a 1:1000 dilution. Nuclear staining was performed using DAPI (DOJINDO Lab, Kumamoto, Japan) at a 1:5000 dilution. Detection of DNA damage was performed using the TUNEL Assay Apoptosis Detection Kit (Biotium, San Francisco, CA, US). Finally, after washing, the sections were decolorized using Rapiclear 1.49 (SunJin Lab Co., Hsinchu, Taiwan).
Measurement of cytotoxicity and inflammatory cytokines
To assess the effectiveness of the culture method using microneedles, lactate dehydrogenase (LDH) and inflammatory cytokines (IL-6, IL-8, IL-10, IL-12, IL-1β, and TNF) in the collected culture medium were measured using an LDH measurement kit (Fujifilm Wako Pure Chemical Industries, Ltd.) and a Cytometric Bead Array (CBA) Human Inflammatory Cytokine Kit (Becton, Dickinson and Company).
Imaging
Nuclei, tropoelastin, and COL VII in fixed and decolorized skin tissue were observed in three dimensions using a confocal laser scanning microscope (A1R, Nikon Solutions, Japan). In unfixed excised human skin tissue, nuclei, cell membranes, ROS, mitochondria, and microtubules were fluorescently labeled with NucBlue Live Cell Stain (Invitrogen, MA, USA), CellMask (Thermo Fisher Scientific), CM-H2-DCFDA (Thermo Fisher Scientific), MitoTracker (Thermo Fisher Scientific), and SPY650-Tubulin (Cytoskeleton, Inc., CO, USA), respectively, at a 1:1000 dilution. Each fluorescent label, along with the autofluorescence of collagen and elastin fibers, was observed using a confocal laser microscope (A1R or AXR, Nikon Solutions). A microscope stage culture device with an attachment for inserting microneedles into skin tissue (TOKAI HIT, Japan) was developed and employed for long-term live imaging.
Statistical analysis
Differences between groups were assessed using Student’s t-test with BellCurve for Excel ver. 7.0 (Social Survey Research Information Co. Ltd., Tokyo, Japan). Results with p values