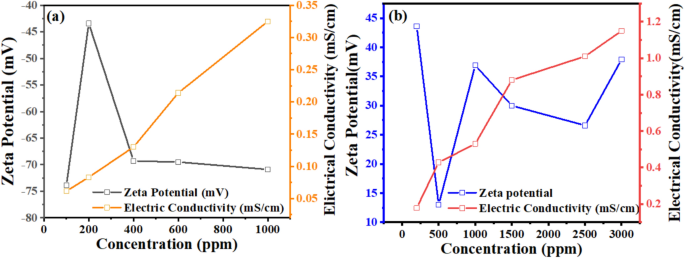
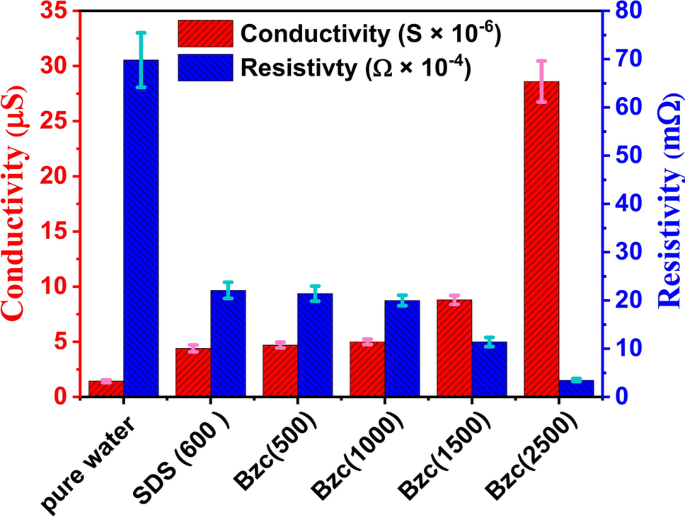
The importance of natural gas in our modern era is significant, as many contemporary approaches focus on storing natural gas in the form of hydrates. Various methods exist for this purpose, but this work specifically concentrates on using cationic surfactants to create gas hydrates. It compares their performance with the well-known SDS commonly used in hydrate formation and storage. In this context, the study also emphasizes analyzing the cationic surfactant solutions used, mainly focusing on the zeta potential and surface tension measurements to determine their role in hydrate formation. Zeta potential was measured to follow the stability of the electrical double layer of surfactant solutions. Zeta potential provides insights into the stability and charge distribution of surfactant molecules in solution, which affects micelle formation46. The surface tension measurements indicate the point at which surfactant molecules begin to aggregate and form micelles, marking the CMC. Together, these measurements offer a comprehensive understanding of surfactant behavior and are crucial for optimizing surfactant concentrations in the hydrate formations. In Fig. 3 and Table 3, the presented data demonstrates the relationship between conductivity and zeta potential measurements used to determine the critical micelle concentration (CMC) of SDS and Bzc surfactants.
The conductivity curve for SDS exhibits a clear inflection point around 600 ppm, indicating the CMC, where the linear increase in conductivity below the CMC transitions to a slower increase as micelles form. Similarly, the Bzc conductivity curve shows that the relationship between electrical conductivity and surfactant concentration is a direct proportional.
The zeta potential curves for both surfactants undergo distinct changes at their respective CMC concentrations, further confirming the onset of micelle formation. Moreover, both surfactants show a decrease in surface tension with rising concentration until reaching a plateau, as illustrated in Fig. 4, indicating the critical micelle concentration (CMC). The Bzc reaches its CMC earlier and at (37 Mol.dcm−3) surface tension compared to Bzc (34 Mol.dcm-3) at 6 °C, which implies that Bzc is more efficient at lowering surface tension. However, Bzc continues to lower surface tension beyond SDS levels, suggesting different micelle formation dynamics or molecular interactions in the solution. These observations provide valuable insights into the self-assembly behavior of these surfactants, enhancing the hydrate formation. Various aqueous solutions: pure water, pure water supplemented with one of the concentrations of SDS (600 ppm)47, and pure water supplemented with different concentrations of Bzc (2500, 1500, 1000, and 500 ppm) were studied in the term of phase equilibrium, induction time, the rate of hydrate formation and NG recovery. Bzc is a thermodynamic promoter that reduces the temperature or pressure necessary for hydrate creation. By incorporating Bzc at different concentrations (parts per million), the phase equilibrium conditions of the NGH system can be modified, leading to an increased storage capacity. This allows for more efficient utilization of the storage medium. Bzc facilitates the formation of NGH by providing suitable nucleation sites and reducing the energy barrier for hydrate crystal growth. This property enables the rapid and controlled formation of NGH during the storage process, enhancing overall storage efficiency. Using Bzc as a thermodynamic promoter allows for reversible hydrate formation and dissociation. This means the natural gas can be easily stored in the hydrate form and released when needed, providing a flexible and controllable storage option. These experiments aimed to understand how these additives affect the formation and dissociation of NGH. Hydrate formation was first initiated in pure water, and subsequent trials were performed in Bzc surfactant solutions at concentrations ranging from 500 to 2500 ppm and in a solution containing 600 ppm of SDS. This comparison allowed for finding changes in formation and dissociation conditions compared to the pure water system. A representative profile showing the relation between pressure and temperature during the hydrate formation in a 600 ppm SDS solution and Bzc (500, 1000, 1500, 2500 ppm) is depicted in Fig. 5a,b. Before nucleation, a slight pressure drop occurs as gases dissolve into the aqueous solution. Upon nucleation, a significant decrease in pressure occurs as the gas becomes encapsulated within the molecular frameworks created by water.
The phase equilibrium of NGH formations
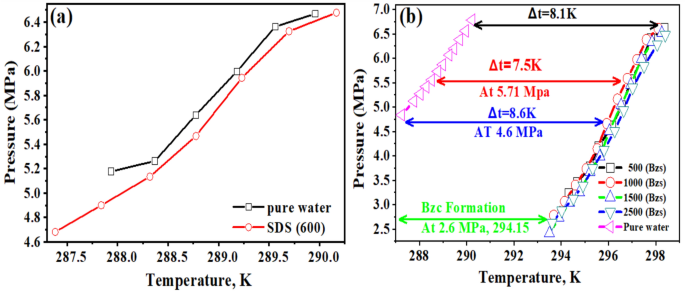
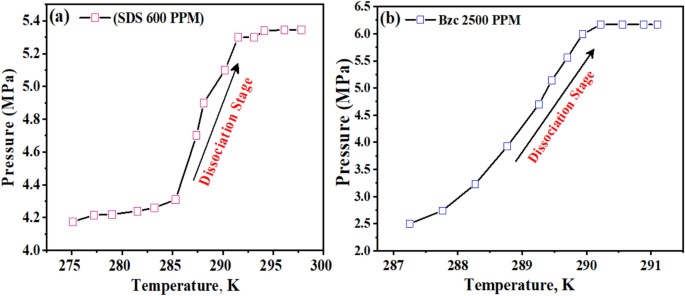
The experiments were carried out under specific conditions: an initial pressure of 6.5 MPa and a temperature of 273.15 K. Details of these experiments can be found in Table 4. The duration of the experiments was 24 h from the start of hydrate formation. The tests focused on the NGH system’s kinetics, specifically investigating the effects of two promoters, SDS and Bzc. To ensure comparable promotional effects on the pure hydrate system’s phase equilibrium, the SDS and Bzc concentrations were carefully selected. Figure 5a,b illustrate the phase equilibrium data for the pure hydrate/semi-clathrate hydrate system in the presence of SDS and Bzc at different volumes.
The variable ΔT represents the boosting effect, calculated by finding the temperature difference between the equilibrium points of a pure hydrate or semi-clathrate hydrate system containing either SDS or Bzc, and the equilibrium point of a pure hydrate system at the same pressure. Comparing the phase equilibrium pressures, the NGH formed in pure water at 289.2 K has a pressure of 5.71 MPa, while the pressure for hydrate formed in the presence of SDS at 289.15 K is slightly higher at 5.74 MPa. This suggests that incorporating SDS does not notably change the phase equilibrium conditions. Other investigators have reached similar findings by examining different systems. The three-phase equilibrium conditions were investigated in the gas mixture containing CH4, C2H6, C3H8, hydrate, and SDS in an aqueous solution48,49. They conducted experiments at four different temperature points (275.2 K, 277.0 K, 278.8 K, and 280.6 K) using an isochoric isothermal dissociation technique and found that SDS does not affect the phase equilibrium. Verrett et al.26 demonstrated that while SDS does not change the bulk solubility of methane at concentrations that support hydrate growth, it does increase the methane concentration in the bulk liquid after nucleation. This indicates that the presence of SDS doesn’t alter the stability of hydrate formation conditions but does enhance the growth rate. Similarly, Gayet et al.50,51 noted that SDS does not affect the methane hydrate equilibrium compared to pure water systems. In summary, the cited research indicates that the introduction of SDS does not significantly impact the phase equilibrium of NGH. Instead, SDS enhances the growth rate without altering the equilibrium conditions. Including Bzc in the water system results in a notable increase in the equilibrium temperature. Specifically, at a pressure of 4.6 MPa, the equilibrium temperature rises to 295.9 K. This corresponds to an approximate temperature increase of 7.8 K under the same conditions. This finding suggests that the presence of Bzc causes a shift in the phase equilibrium curve of NGH in an aqueous solution, leading to a decrease in pressure and an increase in the equilibrium temperature (Δt = 8.6 K). Bzc is an organic compound that is heterocyclic and soluble in water. It readily forms structure II (sII), which hydrates with water in the form (51,264) of crystal structure, as it looks like the TBAB compound. From these observations, it can be concluded that the Bzc acts as an effective thermodynamic promoter. Its presence facilitates the formation of hydrates at lower pressures, which may be beneficial for storing natural gas in hydrate form. This is to be demonstrated in Fig. 5b.
NGH induction time
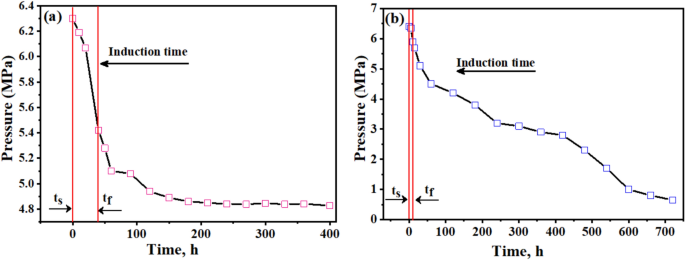
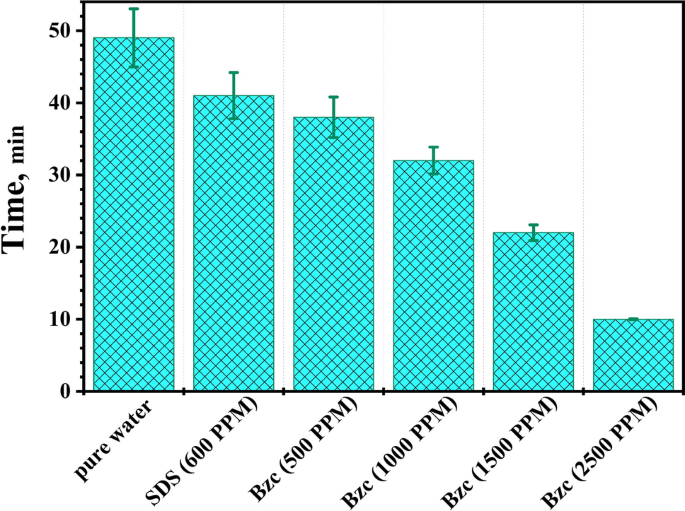
In the context of gas hydrate formation, induction time refers to the delay between the initiation of the experimental conditions (e.g., pressure, temperature) and the onset of hydrate nucleation and growth. The induction time is an essential parameter in understanding the kinetics of hydrate formation, as it represents the initial period required for the system to reach the necessary supersaturation and form the first hydrate crystals13. The induction time can be influenced by various factors, such as the driving force for hydrate formation (pressure and temperature conditions), the presence of promoters or inhibitors, the mixing and agitation of the system, and the availability of nucleation sites. Measuring and analyzing the induction time can provide insights into the mechanisms and kinetics of hydrate formation, which is crucial for understanding and optimizing gas hydrate processes 2. Induction time refers to the period before a noticeable quantity of hydrate phase becomes apparent or until a measurable amount of hydrate-forming gas is consumed 1. Figure 6a,b show the variations in pressure and temperature over time through the hydrate formation in 600 ppm SDS solution with varying Bzc concentrations as the temperature drops by up to 6.7 K. The induction time, defined as the period between the start of the experiment (ts) and the onset of hydrate formation (tf), is reduced when SDS is added compared to pure water, where the induction time is recorded at 49 min. For instance, with 600 ppm SDS in water, the induction time is reduced to 41.3 min.
As the SDS concentration in water increases, the induction time decreases further. As mentioned before, this reduction in time due to SDS has also been documented52,53. With the supplement of Bzc to the water system, the induction time is further reduced by increasing its concentration. As shown in Table 4, the induction time was 37.5, 3.4, and 9.9 min against concentrations of 500, 1000, 1500, and 2500 ppm, respectively. Regarding the effect of electrical conductivity on the formation time of gas hydrates and the equilibrium state of the hydrates, high electrical conductivity facilitates faster transfer of charges and ions within the material. Furthermore, leads to an increase in the formation rate of NGHs. Materials with high electrical conductivity, such as salts and metals, help to speed up the formation of NGH compared with low-conductivity materials15,46. Table 3 shows the data obtained from measuring electrical conductivity and zeta potential at concertation of Bzc and comparing it with pure water and SDS. The measured conductivity and resistivity of different dosages of Bzc, SDS (600 ppm), and pure water were illustrated in Fig. 7. To ensure data reliability, the resistivity and inverse of every prepared solution were measured five times. The error values, resistivity (light blue), and conductivity (purple) were inserted as error bars in the figure. The data indicates that the maximum error reached 9% for the resistivity of pure water, while the minimum error was approximately 3% for the Bzc solution at a concentration of 2500 ppm. Otherwise, Electrical conductivity affects the strength of the hydrogen bonds in the crystal structure of NGH. tend to stabilize the crystal structure of the hydrates and increase their stability10,54,55. This stabilization of hydrate structure is attributed to these materials can enhance ionic mobility, promote favorable electrostatic interactions, improve thermal management, improve heat transfer, and stabilize phase behavior. Moreover, conductive materials can influence the hydrogen bonds within the gas hydrate lattice by modifying the electronic environment within and around the hydrate structure. These materials can enhance the alignment and distribution of water molecular dipoles, which strengthens dipole–dipole interactions critical to maintaining the integrity of the hydrate lattice. Additionally, the presence of these materials can lead to localized polarization effects and increase the strength of van der Waals forces. By stabilizing these intermolecular forces, conductive materials help to reinforce the crystal lattice, reducing the likelihood of structural disruptions and promoting a more robust and stable hydrate framework under varying environmental conditions. These factors collectively contribute to the overall stability and the reduction of induction time hydrate formation10,56.
This leads to an expansion of the thermal stability region of NGH at certain temperatures and pressures. Therefore, increased electrical conductivity helps to stabilize the state of NGH and broaden the equilibrium conditions specific to them57,58.
Figure 8 illustrates how introducing varying amounts of Bzc and SDS at a concentration of 600 ppm affects the induction time. Based on the obtained data, the error bars show that the maximum value is 8.6% for pure water, while the minimum value is 5.4% for Bzc (2500 ppm). This comparison reveals that one promoter is more effective at improving the induction time than the other.
NGH formation among Bzc and SDS
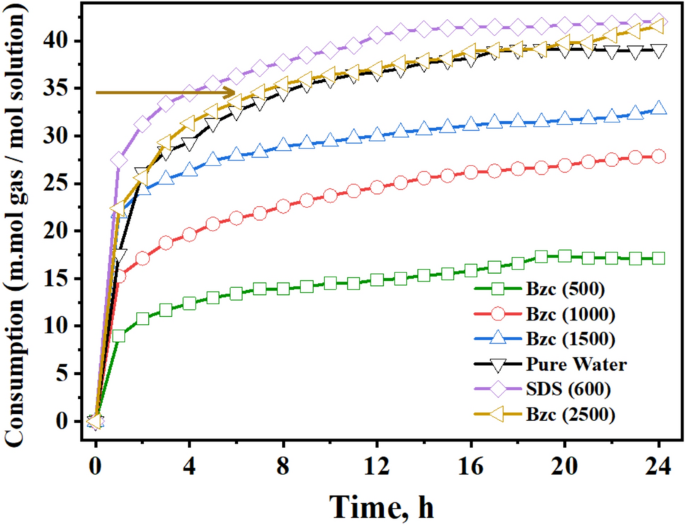
Research has been conducted on the kinetics of the NGH formation using Bzc aqueous solutions at various concentrations (500, 1000, 1500, and 2500 ppm), as shown in Fig. 9. This figure shows the amount of the gas consumed per mole of water through (24-h) the period of hydrate formation, with t = 0 marking the onset. Notably, the 2500 ppm Bzc solution demonstrated the highest gas consumption among the other concentrations.
By increasing the concentration up to 2500, the number of mole gas consumed in the hydrate was being to 0.04211 mol; otherwise, at the concentration of SDS at 600 ppm, the number of moles was 0.4255 mol as reported before. This means that the Bzc exhibited a close number of gas consumption in the hydrate formation and with the SDS. During hydrate formation, the gas consumption curve levels off after a few hours, indicating no further hydrate formation. This rise in gas consumption is due to Bzc or SDS supplements.
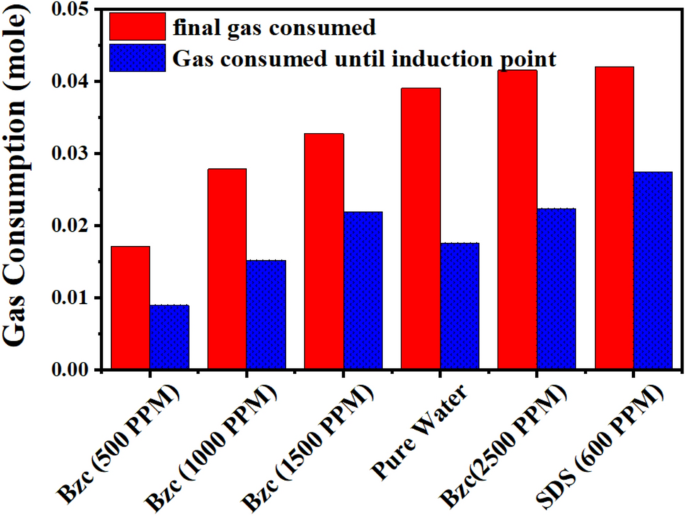
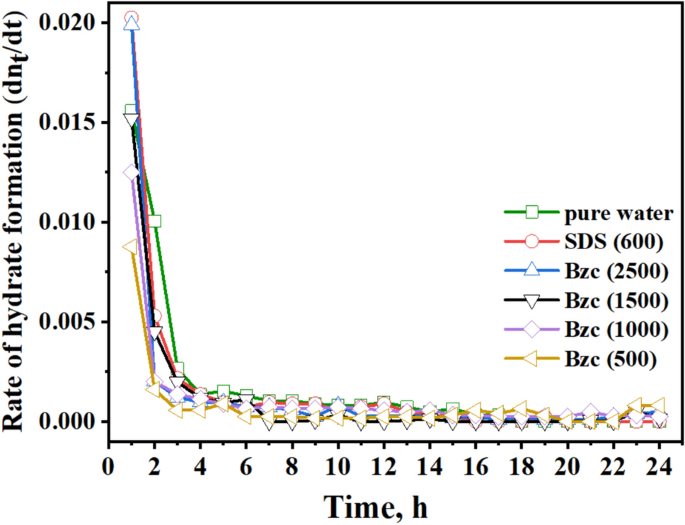
Figure 10 compares the gas consumed from the nucleation period to the final gas uptake. By analyzing the data, one can conclude that the minimum gas consumption until the nucleation, after which it increases rapidly, as reported elsewhere26. One notable benefit of utilizing Bzc is its ability to decrease the induction time relative to pure water and SDS by facilitating faster nucleation (refer to Table 4). Figure 11 illustrates the rate of NGH formation at various concentrations of Bzc aqueous solution, alongside a comparison to pure water and pure water containing SDS (600 ppm) aqueous solution.
In the early stages of the hydrate formation experiment, spanning the first three hours, the SDS-based system demonstrated a rapid hydrate growth rate, followed by Bzc (2500 ppm), pure water, Bzc (1500 ppm). The hydrate formation rate was tested with Bzc at 1000 ppm and then at 500 ppm. However, the rates for Bzc at 2500 ppm, pure water, and pure water with SDS were nearly identical, gradually slowing to zero by the final of the (24-h) hydrate formation test. When comparing the rate of NGH formation among Bzc, SDS, and other systems, it was observed that the systems with SDS at 600 ppm and Bzc at 2500 ppm showed the highest amount of gas consumption per mole of water in the NGH system opposite to other concentrations. Overall, using Bzc in an aqueous solution appears to enhance gas consumption. These findings indicate that further investigation is needed to fully understand the role of surfactants and various promoters in hydrate formation.
NG release through dissociation stage
The dissociation behavior of NGHs was investigated in the presence of two common additives, (SDS) and (Bzc), through pressure–temperature (P–T) measurements1. The SDS-containing system exhibited a distinct change in the slope of the dissociation curve in Fig. 12, suggesting a potential phase transition or structural alteration within the hydrate structure59,60.
In contrast, the P–T profile of the Bzc–NGH system showed a smoother, more continuous dissociation characteristic. These differences in dissociation patterns can be attributed to the interactions between the guest gas molecules and water molecules and the respective additive molecules within the hydrate lattice61. The findings contribute to understanding how the presence of different additives can significantly influence the stability and dissociation properties of NG hydrates. This is crucial for optimizing gas storage, transportation, and recovery processes and developing efficient hydrate management strategies.
Mechanism of NGH formations
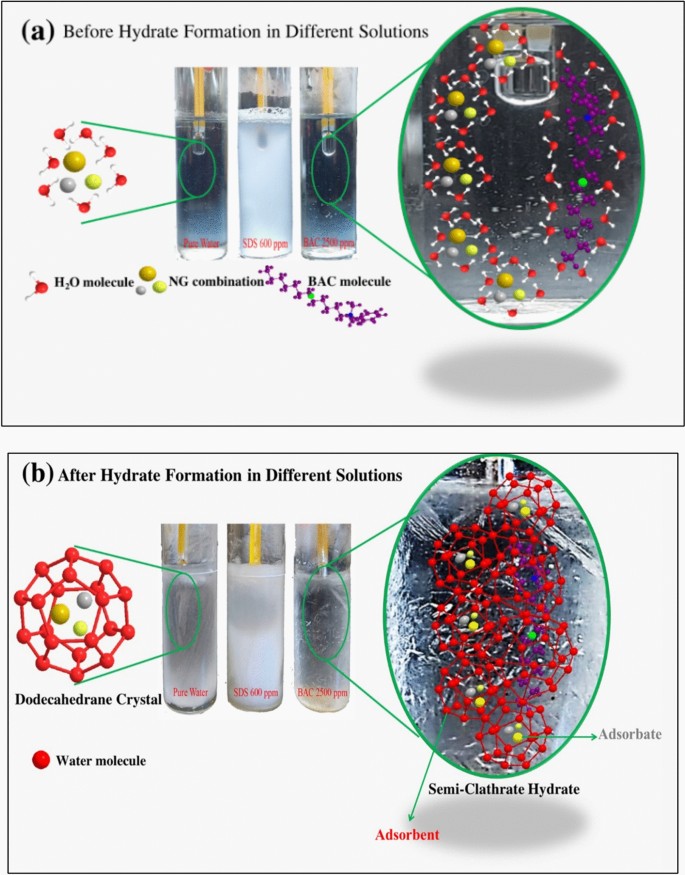
Natural gas consumption within the hydrate structure relies on the adsorption process between the clathrate (micelles and hydrate structure), which acts as the adsorbent, and NG molecules, which act as the adsorbate. This process can occur through one of the following cases: 1) Hydrogen bonding forms bonds between the absorbent and the adsorbate. 2) Adsorption by electron polarization occurs when the electron-rich sites on the adsorbent’s aromatic nuclei attract the positive sites of hydrogen atoms on the adsorbate (NG). 3) Adsorption by dispersion forces involves London-Van der Waals forces, acting between adsorbent and adsorbate, which are stronger with higher molecular weight adsorbents among the adsorbate (NG). Hydrophobic bonding occurs after the initiation of the formation of hydrates, and the first stage of NG adsorption is carried out (named as solid phase).
The continuous adsorption between the NG and the accumulating NG in the clathrate may be occurring by the hydrophobic bonding to continue the process. The mechanism of NGH formation, as illustrated in Fig. 13, demonstrates the influence of surfactants like SDS (600 ppm) and Bzc (2500 ppm) on hydrate stability and nucleation. Before hydrate formation, natural gas molecules are enveloped by a layer of water molecules, with surface-active agents strategically located at the boundary between the gas and liquid phases, thereby enabling molecular interactions. Post-hydrate formation reveals a distinct structural organization where water molecules form a pentagonal dodecahedral lattice (512) around the gas molecules, particularly in the presence of surfactants35. This results in semi-clathrate hydrates, enhancing NGH stability and reducing induction time compared to pure water systems. The surfactants improve gas incorporation and hydrate formation efficiency by promoting nucleation at lower energy thresholds, which is critical for NG storage and transportation applications. This work represents an early-stage investigation focused on using Bzc to solidify natural gas in hydrate form. Compared to the NGH formation system with pure water, the new promoter (Bzc) eased the thermodynamic conditions required for NGH formation and significantly enhanced the formation kinetics and gas uptake. The importance of this research lies in exploring new potential semi-clathrate hydrates to improve NGH storage and developing practical approaches for implementing NGH formation under optimal conditions. After analyzing the results obtained from the measurements of electrical conductivity and zeta potential for surfactant SDS and Bzc, it became clear that the zeta potential signal, which indicates the stability of the electrical double layer of the solutions of the two surfactants, was higher for the Bzc than for SDS. Additionally, the electric charge on the surface-active group of the surfactant molecule was greater in Bzc than in the SDS. Primarily, this is done when focusing on the critical micelle concentration of both surfactants. This indicates that the Bzc is more stable in its solutions than the SDS, especially at the critical micelle concentration.
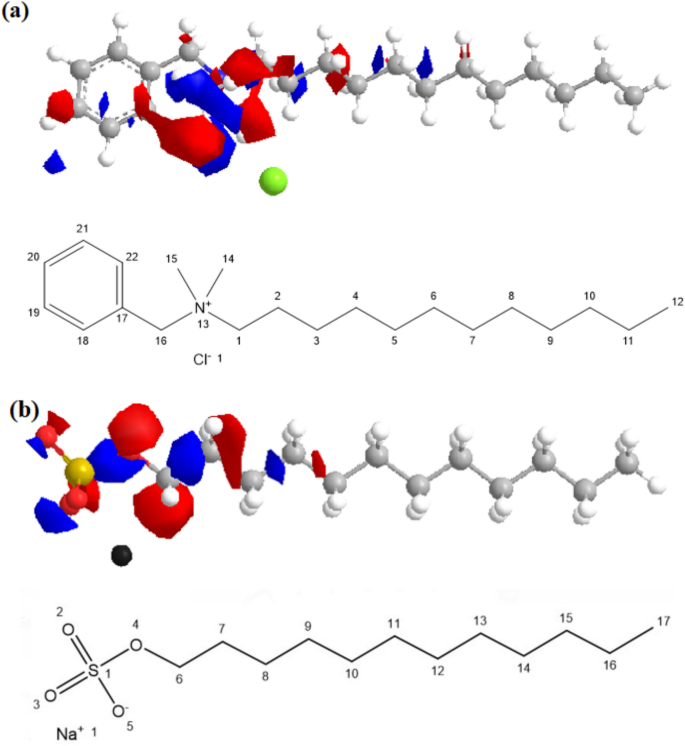
The positive and negative charges are calculated according to the extended Huckel Theory, and their values are listed in Table 5. The obtained data showed that the net negative charge value, which is with Bzc, was − 1.711 eV. Meanwhile, the net charge value of SDS was -0.71; this finding explained why Bzc has more ability to hydrate formation, minimize the induction time, and maximize NG recovery among the SDS. The electronic charge distribution over the Bzc and SDS structure and the atoms within two compounds were numbered IUPAC system as shown in Fig. 14. The distortion in the chemical structure does not accurately reflect its spatial framework configuration. Instead, this distortion arises from the charge distribution across the molecular bonds. Specifically, it indicates the type of charge—negative or positive—dominating various atoms within the Bzc molecule, as according to the extended Hückel theory, where red and blue colors represent partial negative and positive charges, respectively.
These electronic and physical properties placed the Bzc at the forefront when it was chosen to form NGHs. It worked to store it more effectively than the SDS, commonly used in this technological field, as shown in Tables 3, 4, and 5 and Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, and 14, the Bzc achieved the best results regarding the induction time taken to form hydrates, phase equilibrium stability, and the temperature and pressure used for its formation, addressing some shortcomings in using the SDS. This confirms that the wisdom of selecting Bzc as the primary material for investigating the kinetics of formation, induction time, and the optimal pressure required for hydrate formation, as well as the process of hydrate dissociation and comparing it with a commonly used material, was successful in addressing the shortcomings in the performance of material SDS.