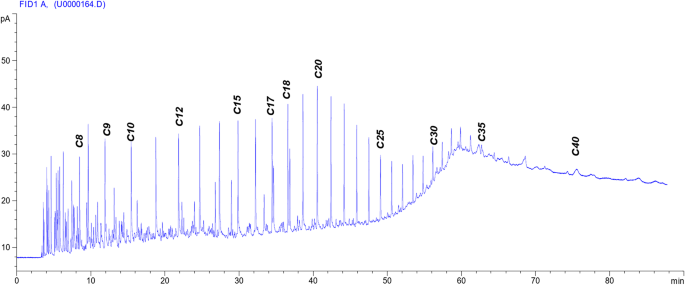
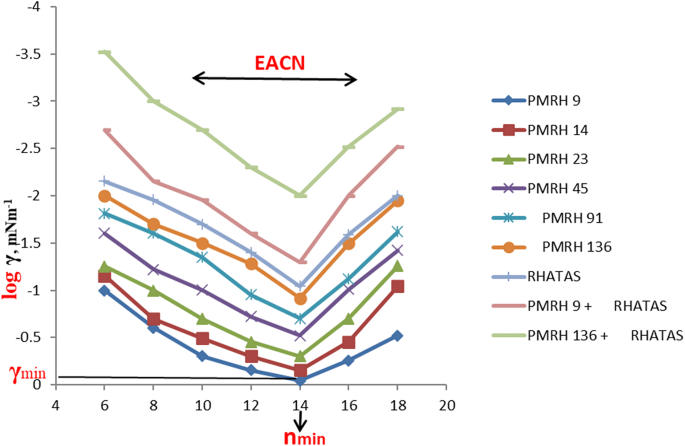
The interfacial tension between crude oil and the injection chemical plays a vital role in the oil recovery process. The undertaken surfactants can reduce the interfacial tension between crude oil and its aqueous solution phase by increasing the ethylene oxide number in the molecule52, as shown in Table 3. The reduction of IFT is a good indication for the formation of water base emulsion (O/W) during the surfactant flooding process, which will help achieve the best possible oil recovery. The IFT was determined against n-alkanes to detect the (n min) (at which the minimum IFT was achieved). It can be identifying the EACN from the V-shaped curves as shown in Fig. 4. The equivalent alkane carbon number (EACN) is shown on the curve lines in Fig. 4. This means that every used surfactant achieved a certain (n min) at n-c14, but at the same time by some different ratio of different n-alkanes up and down the (n min ) can detect the equivalent alkane carbon number (EACN) of the used crude oil. If the used surfactant exhibited a minimum tension in the range of the EACN, it should be pronounced an emulsion (O/W) during the EOR process. By analysis of the GC chromatogram (Fig. 1.), it was found that the total molecular weight of the wax and paraffin of the used crude oil was about 14 (the main soluble hydrocarbon). This foundation is very important to detect the (n min) and the EACN of the used surfactant with the used oil. These factors are considered promising parameters during an investigation of surfactants in chemical oil recovery. The surfactant in this study exhibited (n min ) at n-c14. The γmin (the minimum interfacial tension at the (n min) was sensitive to the ethylene oxide number (EO). The γmin was measured by an increase of the EO number as shown in Fig. 4. The PMRH136 exhibited the minimum value of γmin (7 × 10− 2 mNm− 1) at n-c14. The more minimum IFT was obtained by the blend of PMRH136 with RHATAS (4 × 10− 2 mNm− 1).
Contact angle and wettability alteration
One important feature that surfactants can provide in a variety of applications is the capacity to alter surface wettability. Enhancing the effectiveness of oil recovery (EOR) requires changing the wettability of reservoir rocks in petroleum wells53 The hydrophilicity of the surfactants used for oil displacement is higher than the lipophilicity. When these surfactants are adsorbed on the surface of the formation rock, they can change the rocks’ wettability, which lowers the contact angle between the rock surface and the formation water. This effect increases the efficiency of displacement and decreases the adhesion work between rock and oil. It is expected that water-wet reservoirs will be a good source for obtaining high oil recovery. By measuring the contact angle, θ, with either water or oil droplets, one can determine the strength of the attraction between the water and oil phases in the three-phase system (water solution, oil, and rock surface). In general, a system is categorized as water-wet when using water droplets if the contact angle is between 0˚ and 90˚. While contact angles above 100˚ represent oil-wet conditions, those between 90˚ and 100˚ represent an intermediate wet regime. Thus, a contact angle of less than 90˚is usually required when using the produced surfactants to improve chemical EOR (Enhanced Oil Recovery)54.
The arising different equilibrium phases of the contact angle are associated with the free interfacial energy. This was explained by some investigators55. The surfactant in the formulation of EOR plays an important role in reducing the tension between the formation water and tested oil, consequently, affecting the contact angle between oil and water at the interface area and making an emulsifier to solubilize the oil in the form of emulsion phase (O/W). So, if the angle of contact in the hydrocarbon system is 3. The data in Table 3, indicate that the used blank oil droplet’s contact angle was 159˚ in formation water, which is noticeably high. The sand model sample thus changes from wetted oil phase to the aqueous phase when the surface charge (γs) changes from negative to positive value. The surface free energy of the used surfactants was positive values as shown in Table 4. The γs increased by EO increase. The minimum γs was obtained with PMRH 9 (0.66 mJ/ m2), meanwhile, the maximum of γs was achieved with PMRH 136 (44.64 mJ/ m2). This result may be due to the increase of the (-O-) bond on the poly oxy alkylene chain acts to rises of γs as the presence of a loin pair of electrons on the oxygen atom. The surfactant decreased the contact angle of oil droplets and interfacial tension at the same time as a result of the decrease of the work adhesion. This finding may explain why the reduction requires work of adhesion to repel the oil droplets away from the rock surface and release them in the form of an emulsion56,57,58.If The contact angle of the water droplet was greater than 100 it was θ = 130.07˚ as in Table 3., suggesting that the rock sample was oil-wet and hydrophobic. The contact angle, however, dropped when the surfactant was added to the formation water at or above the increase of the critical micelle concentration (CMC), signifying that the tested model changed from an wetted oil to an aqueous phase.
These variations in the contact angle and interfacial tension suggest a reduction in the capillary forces, which are responsible for passing the liquid through the openings of the small pores. By lowering the energy required for adhesion, the reduction of capillary forces is essential for improving the oil recovery. Consequently, this facilitates the process of removing oil droplets from the sand model surface and releasing the hydrocarbon as an emulsion phase. The efficiency of oil recovery in the reservoirs that naturally have a preference for oil (oil-wet reservoirs) can be increased by changing the wettability of porous reservoir rocks to become more water-attracted (water-wet). By making this change, the contact angle value (θ) is guaranteed to stay at or below 90˚ degrees (θ 42. The undertaken surfactants exhibited contact angles as; 41, 36,30, 25, 19, and 12 against the PMRH 9, PMRH 14, PMRH 23, PMRH 45, PMRH 91, and PMRH 136 respectively. While the contact angle of the cationic surfactant was 22˚.When the anionic surfactants were blended with the cationic, the contact angles became 15˚, and 12˚, for the blends (PMRH 9 + RHATAS) and (PMRH 136 + RHATAS) respectively. This change in the contact angle indicates to shift in the phase from wetted oil phase to the aqueous phase of the rock sample. This reduction in the contact angle facilitates the displacement during the oil recovery process .With the increase in the ethylene oxide content. The decrease in the contact angle as shown in Table 3, indicates improved surface wettability. This decrease in the angle of contact may be attributed to the affinity of the used surfactant to reduce the interfacial tension between the water and the oil at rock pores and surfaces. As a result, these surfactants exhibited enhanced wetting properties.
Work adhesion
In many surfactant applications, the wetting process is a critical character. The reduction of surface tension brought about by a surfactant has an impact on the contact angle (̨θ) of a liquid with a solid surface (oil, formation water, and rock). The contact angle is also affected by the solid surface energy and solid-liquid interfacial tension in addition to the tension of the liquid. The position and orientation of surfactant molecules at the solid-liquid interface have an impact on the adhesion process. The variation between cohesion work (Wc) and the work of adhesion of solution to the surface (Wa) can be used to inform the wetting process. The Adhesion work (Wa) and contact angle (θ) are dependent on the surface tension of both liquid and solid surface tension. The residual oil saturation dropped and the displacement mechanism became simpler as a decrease of contact angle between the fluid, oil, and rock system. For the surfactant molecules present in the flooding process to adsorb in the oil/rock interface layer, the contact angle increases with the oil and decreases with the surfactant liquid/rock. The amount of oil collected increased as a result of the oil starting to flow on the surface of the sandstone or in the pores and becoming soluble as an oil-in-water emulsion. The adhesion work was computed and reported in Table 4. The Younge-Dupre equation was used to compute the work adhesion.
$${W_a} = {\gamma _L}\left( \rm{{Cos\theta}+ 1} \right)$$
(11)
The young-Dupre equation, which links the work adhesion to the wetting liquid`s surface tension, invariably result in more adhesional wetting ( γL) and contact angle at the solution interface ( θ )59,60. From the data in Table 3, it was found that the increase of the contact angle (decrease of Cos θ) reflects an increase in the solution surface tension. When the contact angle is 180˚, which is never reached, the driving force of adhesional wetting is equal to zero and can never be negative. The term “cohesion work” (Wc) refers to a liquid self-adhesion.
$${{\rm{W}}_{\rm{C}}}{\rm{ = 2}}{{\rm{Y}}_{\rm{L}}}$$
(12)
When, if Wa > Wc, the spreading coefficient forms a thin layer on the sandstone by spreading on its own, if Wac, the spreading coefficient is negative, if θ is greater than zero, and the liquid produce droplets or lenses with a restricted contact angle rather than spreading across the substrate (sandstone). The spreading coefficient is zero when the work of adhesion and the work of cohesion are equal.
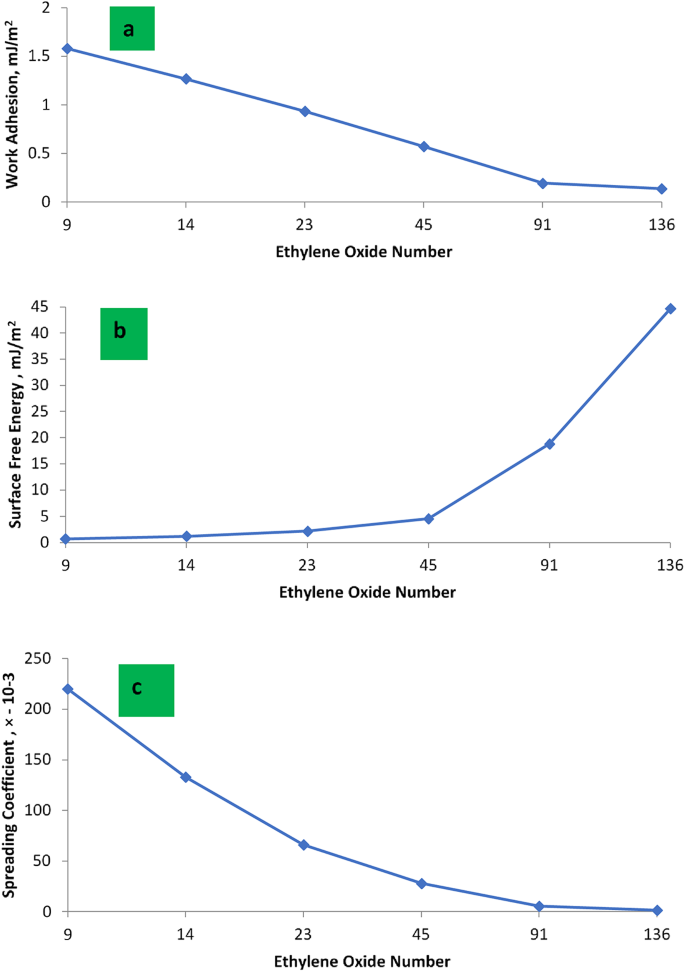
From the data of (Wa) in Table 4, it was found that the Wa decreases as the EO number increases as shown in Fig. 5. The value of Wa for PMRH 9 was 1.579 mJ/m2, whereas it was 0.138 mJ/m2 for PMRH 136. The blend between the cationic surfactant and nonionic (PMRH 136) exhibited positive synergism and pronounced Wa equal to 0.079mJ/m2. It seems that the EO content plays an important role in decreasing the Wa which eases to repel the oil droplet from the surface.
Spreading coefficient of surfactant on the rock surface
The spreading coefficient, which is a mathematical expression for the variation in the adhesion work and the work of cohesion, describes how liquid spreads over a solid surface. For the oil to naturally spread across the sandstone rock`s surface (or model), reduce the system`s surface free energy, which depends on a decrease in the area at the interface. The saturation of the rock by oil is in equilibrium with interfacial tension. Spontaneous spreading is possible if the spreading coefficient is positive and it is vice versa if it is negative. The spreading coefficient of surfactant molecules on the surface of the sandstone was determined using Eq. (13).
$${{\rm{S}}_{{\rm{coff}}}}\,{\rm{ =\,}}{{\rm{\gamma }}_{\rm{L}}}\left( {{\rm{Cos}}\theta{\rm{ }} – 1} \right)$$
(13)
The spreading coefficient (Scoff) is equal to the difference between the work adhesion of liquid and its work of cohesion for the sandstone model.
$${{\rm{W}}_{\rm{a}}}{\rm{-}}{{\rm{W}}_{\rm{c}}}{\rm{\,=\,}}{{\rm{S}}_{{\rm{coff}}}}$$
(14)
Where Scoff is the spreading coefficient, γL is the IFT and θ is the contact angle (finite), (Cos θ −1) is always negative and resulting Scoff negative value as shown in Fig. 5. If the contact angle is 0, the spreading coefficient may be zero or positive. In both cases, total spreading wetness, take place. It can be used spreading coefficient as an index to measure wettability because the spreading coefficient is positive and rises with increasing wettability51,61.
From the data in Table 4, it was found that, the spreading coefficient of the (PMRH 136 + RHATAS ) equal − 0.87 × 10 − 3. Meanwhile, it was − 15 × 10− 4 for the individual surfactant PMRH136 .
Surface charge energy
The surface-free energy was calculated using Eq. (15).
$${{\rm{\gamma }}_{\rm{L}}}\,{\rm{cos \,\theta = \theta \,}}{{\rm{\gamma }}_{\rm{S}}}\,{{\rm{\gamma }}_{{\rm{SL}}}}$$
(15)
Where γSL the interfacial tension of the system, γL the surface tension of the liquid (m N/m), and γS the surface free energy of the rock, by rearranging Eq. (16)51.
$${{\rm{\gamma }}_{\rm{S}}}{\rm{\,=\,}}{{\rm{\gamma }}_{\rm{L}}}\,{\rm{cos\, \theta /\;\theta\,}}{{\rm{\gamma }}_{{\rm{SL\;\;\;\;\;\;\;\;\;\;\;\;}}}}{\rm{mJ/}}{{\rm{m}}^{\rm{2}}}$$
(16)
The maximum value of the surface charge energy was obtained with the PMRH136 (44.64mJ/m2). Meanwhile the minimum value was achieved by the PMRH9 (0.66064mJ/m2 ). However, the lowest interfacial tension (4 × 10− 2 mNm− 1) was exhibited against the blend ( PMRH136 + RHATAS). As a result of decreasing the tension, this blend achieved a surface charge energy equal (72.68 mJ/m2). It can be concluded that the increase of ethereal bond (-o- ), increases the surface charge energy. The increase of oxygen bonds along the ethylene oxide chain in the surfactant molecules plays an important role in increasing the surface charge energy as shown in Fig. 5. This means that the surface charge energy is directly affected by the surface of the liquid and the contact angle, which provides that, the surfactant chemical structure is very important in the EOR.
Rheological properties
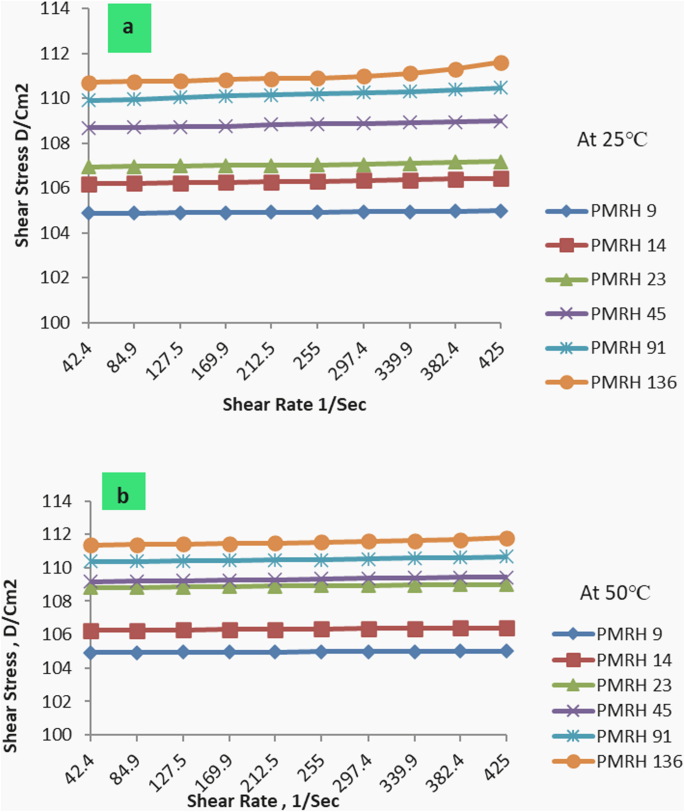
The rheological properties of the synthesized nonionic surfactant at a salinity of 50.000 ppm were determined at 25 and 50°C. The Herschel-Bulk ley equation was utilized to analyze the flow curve62.
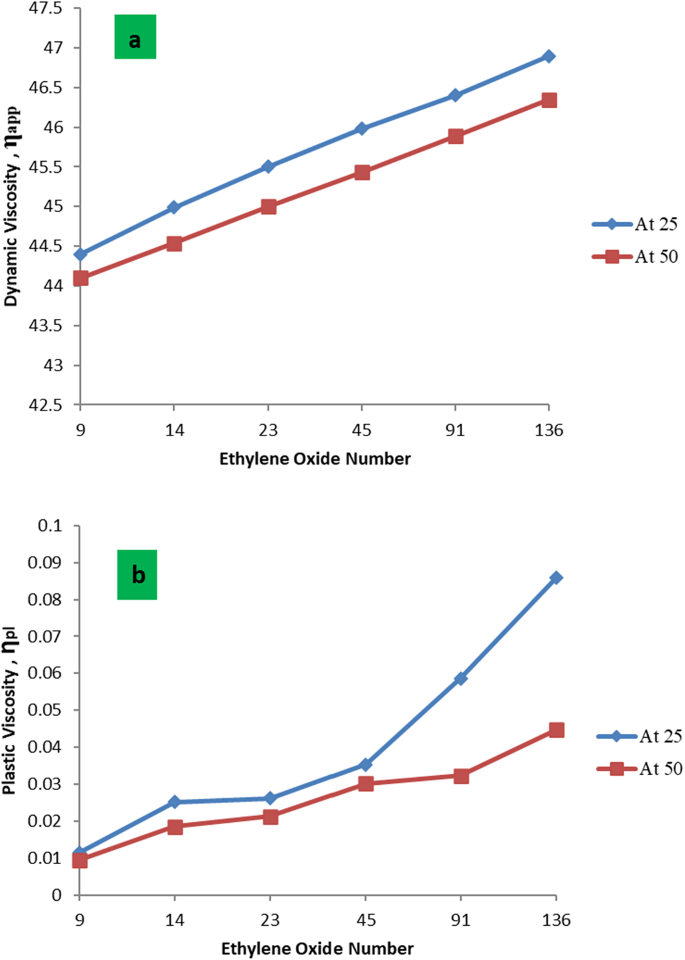
The effect of the used nonionic surfactant on the rheological behavior of formation water at different temperatures was listed in Tables 5 and 6. It was found that the values of apparent viscosity ( ηapp ) and plastic viscosity ( ηpl ) of the PMRH 9, PMRH 14, PMRH 23, PMRH 45, PMRH 91, PMRH 136 increase with increasing of ethylene oxide number and decrease with increasing temperature as shown in Fig. 6. The yield value (ƮB) increases with increased ethylene oxide number and temperature as shown in Table 5; Fig. 7.
One can conclude that these surfactants not only reduce the interfacial tension but also increase the viscosity of their solutions. This finding gives these surfactants the privilege to play two roles in the EOR process. The first role is to minimize the IFT, which serve to repel the emulsion-forming oil droplet. The second role is to increase the viscosity of the chemical flooding solution which may act as sweeping of oil and emulsion from the pores, consequently enhancing the recovery factor. This observation may be pronounced as the result of an increase of ethylene oxide chains that are coiling in the solution, consequently, the viscosity increases. Enhanced Oil Recovery Factor of the Surfactant Flooding.
The nonionic and cationic surfactants were used in chemical flooding formulations to assess the effectiveness of oil recovery. To change the wettability of the rock surface, the flooding approach depends on choosing the ideal surfactant concentration while accounting for variables including critical micelle concentration (CMC), interfacial tension (IFT), and contact angle (CA). These elements have a great impact on how the flooding process turns out.
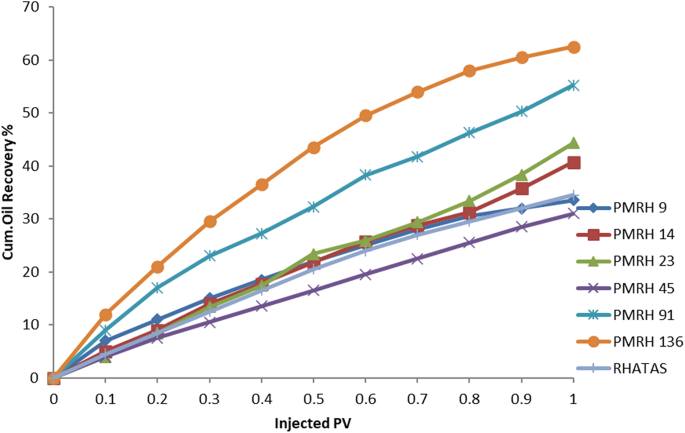
The injected pore volume of a sand-packed model was used for various sets of flooding experiments for the nonionic and cationic surfactants at somewhat higher CMC concentrations, temperatures (50 °C), and salinities (50 × 103 ppm). The trapped oil in the pores is released upon flooding with a surfactant solution due to a reduction in the IFT between the oil and the injecting surfactant solution. To interact with the trapped oil, it can lower the IFT, decrease the wettability of the rock, solubilize the oil by generating an oil-in-water emulsion, and raise the recovery factor (RF). By analysis of these factors, the (PMRH 9, PMRH 14, PMRH 23, PMRH 45, PMRH 91 PMRH 136, and RHATAS) exhibited high performance and favorable results. The process of flooding in a one-dimensional model with sandstone under reservoir conditions that were simulated was investigated through experiments. Two distinct methods—a secondary oil displacement (2ry recovery) and a tertiary oil displacement technique (3ry recovery), which involved two processes overall—were used to establish the oil recovery factor. After the sand pack was saturated with crude oil, brine was injected into it in the first stage, which is equivalent to the secondary recovery technique (2ry recovery). The remaining oil was left in the rock, but some of it was successfully extracted. High interfacial tensions alone—no chemical interaction—caused the oil to be displaced by the formation of water.
In order to recover the residual oil in the sand pack model, a tertiary recovery method (3ry recovery) has to be used in the second step. During the flooding process, this technique involves injecting solutions of surfactants (surfactants dissolved in formation water). These solutions reduced the interfacial tensions between the oil and formation water fluids and increased sweeping efficiency. The amount of recoverable oil rose as a result of increased ethylene oxide content. The oil recovery volume recovered in each step (secondary and tertiary oil displacement procedures) was added up to determine the total oil recovery factor (RF), which can be reported as a percentage in milliliters (ml) or as a percentage of the original oil in place (OOIP).
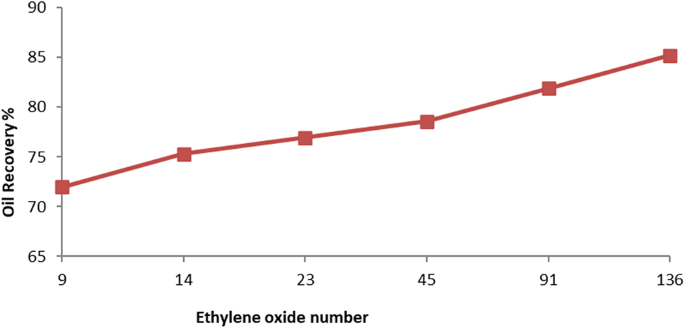
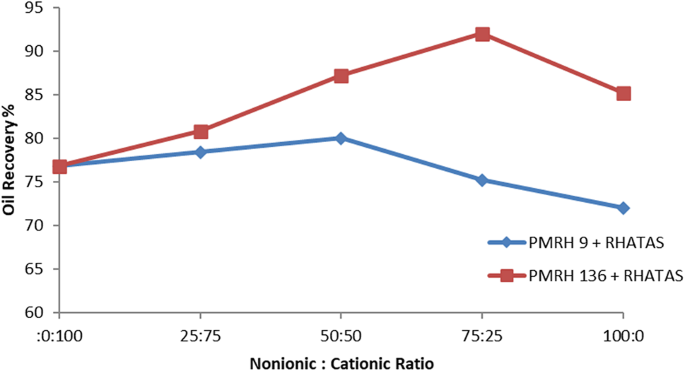
The total recovery factor determined by secondary and tertiary recoveries is denoted by RFTotal. According to results, the overall recovery factor for PMRH 9, PMRH 14, PMRH 23, PMRH 45, PMRH 91, PMRH 136, and RHATAS was 72%, 75.3%, and 76.94% OOIP, 78.6%, 81.9%, and 85.2% OOIP, respectively, and 76.8% OOIP for RHATAS as shown in Table 7.And Figs. 8 and 9. According to these results, the recovery factor increases as the ethylene oxide number increases as shown in (Fig. 10). The maximum recovery for individual used surfactant obtained PMRH 136 (85.20%), and the lowest recovery achieved by PMRH 9 (72%). However with blends between the RHATAS and PMRH 9 and with PMRH 136 at various ratios from (nonionic: cationic) surfactants, it was found that the blend (PMRH 136: RHATAS) pronounced the highest oil recovery factor at a ratio (75:25). As shown in Table 8; Fig. 11.The total recovery factor for this blend arrived 92%.
This result was obtained as the result of mixing surfactant at a certain ratio, a good micelle should be formed, and maximum reduction of IFT was achieved lowest contact angle. These factors make the maximum solubilization of the oil throughout the flooding procedure, furthermore, the RF increases.
Mechanism of enhanced oil recovery method42
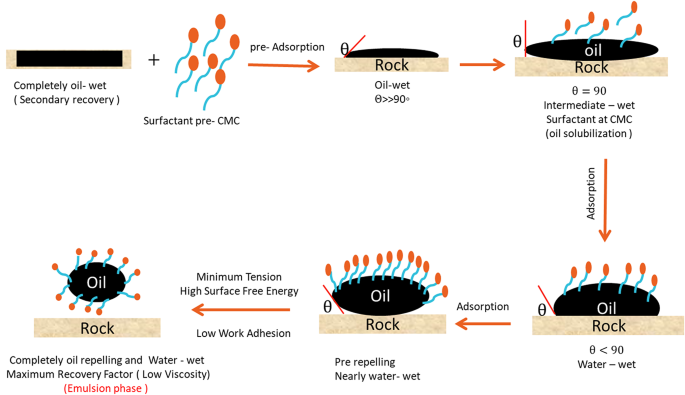
In this work the mechanism of enhancing the recovery factor on the light of changing wettability, contact angle, IFT, work adhesion, spreading coefficient, and surface charge energy is described in different phases as shown in Fig. 12. At the surfactant phase, the rock (sand backed model) is completely oil-wet in the presence of surfactant, the molecules are adsorbed continuously up to make completely adsorption which have been achieved at the CMC. The adsorption process passes via the adsorption of monomeric molecules, then at the pre-micelle phase up to the complete micelle form. The oil droplet is in complete contact with the oil-wet rock surface, by introducing the surfactant pre-CMC will affect the contact angle, where, the rock surface that is moist with oil has the oil droplet adhered to it (θ>>90˚). At the solubilization phase, the trapped oil surface is surrounded by a monomeric adsorbed layer created by the surfactant (on the surface of oil, surfactant molecules begin to adsorb ) at this point the surface is still oil wet but the contact angle somewhat reduces ( θ = 90˚), the complete monolayer of the adsorbed surfactant is formed at the CMC of the surfactant. The surfactant molecules reduce the IFT by penetrating the rock-oil contact. Therefore, by forming a persistent layer between the rock surface and the oil droplet, the penetrating surfactant molecules reduce the contact angle further (θ ≤ 90˚), which is called intermediate wettability. At pre- repelling phase ( nearly water-wet ), The IFT and work adhesion will reach to minimum value and high surface free energy at the final stage, and the contact angle is going very low (θ