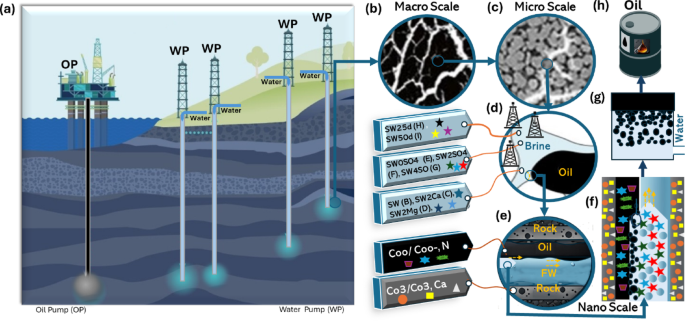
Typically, an increase in salinity, in the presence of asphaltene-containing oil with basic properties, leads to an increase in the IFT of the solution. Conversely, a decrease in IFT is observed in the presence of acidic oil. This phenomenon depends on the surface charge of asphaltenes and the ionic strength of the solution, as determined in this study using zeta potential analysis. The quantity of n-heptane used for asphaltene precipitation from crude oil significantly affects their surface properties. Asphaltenes precipitated with low volumes of n-heptane exhibit different characteristics from those precipitated with higher volumes. As outlined in Section S1 in the Supplementary Information, asphaltenes were extracted from a heavy oil sample by mixing a specified quantity of heavy oil with n-heptane at a ratio of 1:40 g/mL, following the standardized ASTM D6560 procedure. The acid and base numbers of the asphaltenes were determined through potentiometric titration. These properties, related to the functional groups containing oxygen and nitrogen heteroatoms, were found to be 3.24 mg of KOH/g and 9.21 mg of KOH/g, respectively. Previous studies28,29,30,31,32 suggest these values vary with the amount of n-heptane used. The results suggest that the asphaltenes are predominantly basic, with a concentration of basic groups approximately three times higher than that of acidic groups.
FTIR spectroscopy was employed to analyze the structure and functional groups of the asphaltenes. The spectra revealed a broad, noisy absorption band between 3907 and 3470 cm−1, attributed to the stretching vibrations of OH groups from surface-adsorbed water (Section S2, Figure S1, Supplementary Information). Bands corresponding to aromatic rings and ether/ester groups were distinctive, while other bands, indicative of saturated fatty acids, confirmed the presence of aromatic rings, aliphatic chains, and carboxylic groups in the asphaltene structure. For zeta potential measurements, aqueous suspensions of asphaltenes were prepared using synthetic oils with heptol volume ratios of 1:5 and 1:40 and 0.2% (w/v) asphaltenes. A 10 mL sample of each suspension was diluted with 100 mL of deionized water containing 0.001 M NaNO₃ to maintain constant ionic strength. The pH was adjusted using dilute sodium hydroxide or hydrochloric acid solutions and conditioned for 20 min under magnetic agitation. If the pH fluctuated during conditioning, it was readjusted.
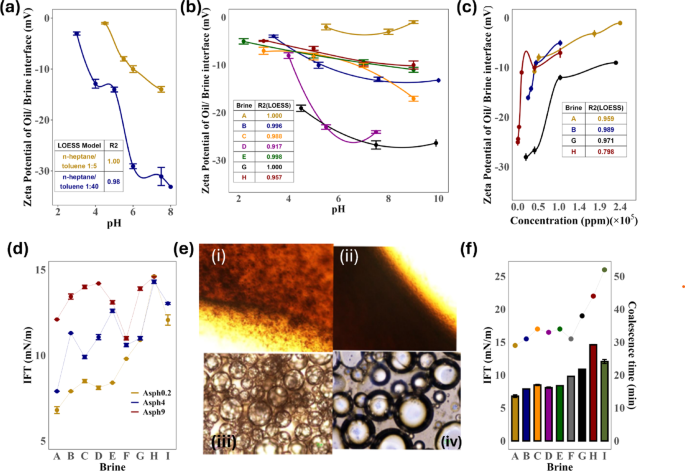
Figure 2 presents the interfacial properties and zeta potential measurements of oil/water interfaces under various salinity and pH conditions. These results provide insights into the effects of asphaltene concentration, brine salinity, and pH on interfacial behavior and emulsion stability. Zeta potential measurements demonstrated that the electrokinetic behavior of asphaltenes in aqueous suspensions depends on the volume of n-heptane used. Asphaltene samples with a 1:5 heptol ratio exhibited an IEP at pH 4.5, while sample with 1:40 ratios had IEPs around pH 3 (Fig. 2a). The surface charge of asphaltenes, whether positive or negative, results from the dissociation of acidic functional groups and protonation of basic groups like carboxylic and pyridine or amin groups. Above the IEP, the negative zeta potential is attributed to the dissociation of acidic functional groups, likely carboxylic groups. Below pH 4.5, the protonation of nitrogen-containing basic groups in the 1:5 (v/v) heptol ratio results in a positive zeta potential.
Oil/water interface measurement results, (a) Zeta potential of O/W interface (heptol with 0.2% asphaltene (1:5 and 1:40 n-heptane/toluene) as a function of pH in the same brine, (b) Zeta potential of oil droplets in FW, SW, SW4SO4 and SW25d brines in variety of pH (1:5 heptol, Asph 0.2% (w/v)), (c) Zeta potential of oil droplets in different brine concentrations and in neutral pH (1:5 heptol, Asph0.2%),(d) Interfacial tension (IFT) of oil/ brine interface at different brine and asphaltene concentration (1:5 heptol, Asph0.2% 4,9), (e) Microscopy image of the oil /brine interface (i), (iii)High coalescence time-high elasticity (less salinity) and increase zeta for LSW in 1 and 500 μm scale and (ii), (iv) Low coalescence time-low elasticity and zeta for LSW in 1 and 500 μm scale and (f) Coalescence time (min at 80 °C) of oil droplet at different brine and heptol 1:5 with 0.2% asphaltene concentration (line plot).
These findings highlight the role of n-heptane volume in determining the electrical surface properties of asphaltenes. The relationship between pH and zeta potential for n-heptane/toluene mixtures (1:5 and 1:40 (v/v)) reveals that higher pH levels result in a stronger negative surface charge. The n-heptane/toluene 1:5 solution consistently exhibited a less negative zeta potential than the 1:40 solution, indicating a lower negative charge density. This difference was more pronounced at higher pH values, which could have implications for oil recovery processes where surface interactions are pivotal. Each sample was measured five times to ensure accuracy. The small error bars denote high reliability in the data. Statistical analysis (Sections S3–S4, Table S1 and Figure S2, in the Supplementary Information) revealed that the zeta potential data for both mixtures were non-normally distributed (p 35,36,37,38,39, the size of asphaltene aggregates in model oil is influenced by the asphaltene concentration and heptol ratio. Larger aggregates (2.5–3 μm) were observed with a 1:5 (v/v) heptol ratio and 0.2% (w/v) asphaltene concentration, closely resembling the aggregates in reservoir residual oil35,36,37,38,39. In conclusion, the heptol ratio (amount of n-heptane to toluene) and asphaltene concentration significantly affect the size, surface charge, and electrokinetic behavior of asphaltenes. Variations in zeta potential can be linked to the abundance of acidic and basic functional groups, aggregate size, ionic strength, and ion types present in the solution.
Zeta-potentials of oil/water interface
The zeta potential at the oil/water interface is influenced by factors such as brine charge (ionic strength), asphaltene charge, pH, and the type of ions present. Additionally, varying the heptol ratio reveals different charges to the oil/water interface. The diverse zeta potentials of crude oil droplets in different brines can be attributed to the combined effects of pH, ionic strength, and specific adsorption of ions or polar components at the oil–water interface.
During the test of the zeta potential of oil droplets in mixed ion brines, a synergistic effect of different ions and pH was evident. As anticipated, given the presence of carboxylic acid groups and phenols in the oil, the surface charges at the oil/brine interface were predominantly negative, diminishing further with decreasing brine salinity. The oil droplet in FW exhibited a slightly negative zeta potential, though close to zero, and only a limited number of points could be measured for FW due to the measurements not stabilizing at higher pH values39. In contrast, the zeta potential values in SW were more negative, approximately around − 12 mV, and further decreased in SW25d (ranging from − 12 to − 25 mV for pH between 6 and 10.5). For SW and SW25d, reducing the pH below 7.0 led to a less negative zeta potential. Additionally, with further reduction of pH to around 3–4, the negative charges at the oil-brine interface were neutralized, causing the zeta potential to approach close to zero values, indicating the IEP. In general, measurements at higher pH levels (exceeding 9–10) were observed to be less stable (see Figures S3–S4 in Sections S5–S6 of the Supplementary Information). The zeta potential is unlikely to be influenced by mineral precipitation, indicating its dominance by chemical interactions at the oil/brine interface involving ions in the brine and the polar functional groups (acidic and basic species) in the oil.
Typically, oil droplets in saline water exhibit a negative surface charge or negative zeta potential for three primary reasons3,22, namely: (1) The adsorption of dissociated weak acids, such as naphthenic acids, from crude oil onto the oil–water interface imparts a negative charge to the droplet surface. These carboxylate anions (–COO−) generated in aqueous environments, especially at higher pH, are a significant source of the negative zeta potential, (2) Negatively charged ions present in the saline water, such as hydroxide (OH−) or chloride (Cl−), can interact with the nonpolar components of the crude oil at the interface. These interactions enhance the overall negative charge on the droplet surface and (3) In saline environments containing multivalent cations like Mg2+ and Ca2+ (as seen in SW2SO4 and SW4SO4 brines), these cations can strongly interact with the polar groups in the oil, such as carboxylates. While these cations typically compress the EDL, they also form specific ion pairs or surface complexes, which can further stabilize or modulate the negative zeta potential at the interface. As the SO42− concentration increases, the zeta potential stabilizes at around -25 mV. This outcome suggests that Mg2+ and Ca2+ ions exhibit a more robust interaction with oil compared to SO42− ions, beyond merely suppressing the EDL.
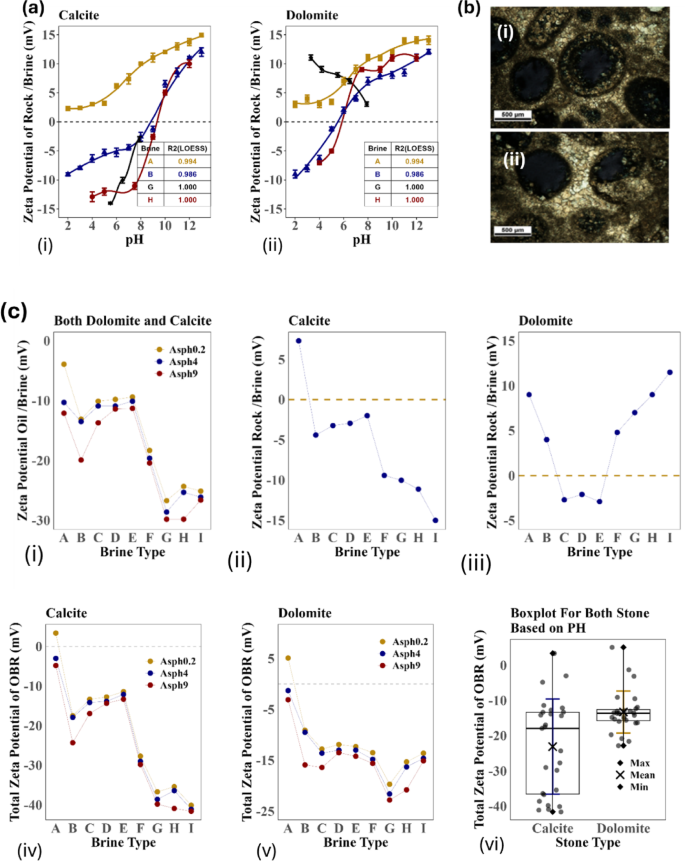
The increase in ionic strength of the brine is known to decrease the magnitude of the zeta potential of crude oil droplets due to the compression of the electrical double layer. Conversely, in reservoir conditions (FW), the carbonate surface typically carries a positive charge, as indicated by the results presented in the following section (water/rock interface section (Fig. 3)). The zeta potential of carbonate rock particles is influenced by factors such as pH, ionic strength, and notably the potential determining ions in the electrolyte solutions due to complex surface reactions involving calcite and dolomite40. The findings reveal that the zeta potential increases with the dilution of SW concentration, reaching approximately -11 mV within the tested range. This aligns with the phenomenon where the zeta potential of colloids at high salt concentrations tends to stabilize at a finite value. The increase in zeta potential in SW and SW25d solutions primarily stems from the suppression of the double electric layer with the rising ionic strength in the solution.
Water/rock interface measurement results: (a) Zeta-potential of (i) calcite and (ii )dolomite particles in FW, SW, SW4SO4 and SW25d as a function of pH and (b) EDL microscopic photos of calcite in (i) FW and (ii) SW25d, (c) Zeta potential (mV) (i) Oil (heptol 1:5)/brine interface(pH = neutral), (ii), calcite/brine Interface (pH = 7), (iii) dolomite /brine Interface (iv) total value of zeta Potential (mV) (ξ oil/brine + ξ rock/brine) for calcite, (v) total value of zeta potential value (mV) (ξ oil/brine + ξ rock/brine) for dolomite and (vi) total zeta potential distribution in both rocks.
The zeta potential measurements of oil droplets highlight the significant influence of ion type on the surface charge of oil droplets in smart water with low salinity. Basic media induce a negative surface charge, whereas high-acidic brine media result in a positive charge on oil droplets, with the IEP observed at around 2–5 for different brines41. Under high salinity conditions, the absolute values of zeta potential approach zero, with the polarities influenced by three factors, namely: (1) the concentration of ions in the brine, (2) pH and (3) the types of ions present. According to Buckley et al.42,43, the oil/water interface is negatively charged due to the dissociation of carboxylic acids. However, at lower pH values, a positive charge is observed, suggesting the presence of basic groups at the oil/water interface. Among the acidic and basic functional groups, naphtenic acids and pyridinic nitrogen bases are considered the most interfacially active. Naphtenic acids constitute a mixture primarily composed of cyclopentyl and cyclohexyl carboxylic acids with a molecular weight ranging from 120 to 700, along with a lower content of other fractions such as carboxy phenols, porphyrins, and asphaltenes14.
IFT and elasticity of the oil/water interface
The IFT at the oil-brine interface is an important factor influencing capillary forces, which in turn dictate the mobilization of trapped oil. Consequently, IFT plays a role in determining oil recovery and shaping production schemes in EOR. However, the reduction of IFT does not exhibit a consistent trend with ions and their concentrations. As observed by Vijapurapu et al.44, there is an initial decrease in brine/crude oil IFT when the brine is diluted to 50% with deionized water, followed by an increase in IFT with further dilution of the brine. The decrease in the work of adhesion resulting from lower oil/brine IFT is helpful for enhancing the mobility of trapped oil. Ions known to reduce IFT at the fluid–fluid interface may induce changes in the viscoelasticity of oil/brine interfaces, wherein the interfacial elasticity can hinder the snap-off of oil droplets. This leads to a more continuous interface that can be more easily swept, contributing to improved oil recovery. Garcia et al.45 investigated the impact of asphaltenes and organic acids on the interfacial viscoelasticity of crude oil/brine and the resulting oil recovery during LSWF. Their findings suggested that although snap-off suppression is associated with viscoelasticity, it is crucial to consider other key factors such as mobility control, wettability, and fluid distributions to gain a comprehensive understanding of the mechanisms involved in LSWF. While it has been widely acknowledged in water flooding that maintaining the connectivity of the oil phase by increasing the rigidity of the oil/brine interface helps prevent snap-off, it was emphasized that excessively high rigidity of the interface is not necessarily required for achieving high recovery. On the other hand, the ultimate rupture of the thin film, facilitating oil droplet coalescence, is closely tied to the rigidity of the film. The results of IFT of oil/brine interfaces at different brine and asphaltene concentrations are shown in Fig. 2d.
As previously mentioned, the following factors are all influential at the IFT of the oil/water interface and elasticity: brine charge (ionic strength), asphaltene concentration, heptol ratio, pH, and the type of ions present. Asphaltene concentration has a significant effect on IFT values. The IFT increases with higher asphaltene concentrations, which is consistent across all brine types.
The IFT in the presence of the asphaltenes decreases as the salt solution concentration increases, and a reverse trend is observed at higher concentrations of the salt solution, and also asphaltene concentration, in agreement with the reported literature on divalent ions46. Addition of salt to water generates electrostatic fields, thus altering the distribution of the polar asphaltenes at the interfaces through the salting-in and salting-out effects47. At lower concentrations of the salt, due to the electrostatic forces, the population of the asphaltenes polar groups at the interface increases, leading the IFT decrease; the mechanism is called the salting-in effect. On the contrary, due to the salting-out effects at higher concentrations of the salt, the salt cations possessing a high concentration at the interface tend to interact with the polar groups of the asphaltenes. It is suggested that the salt cations, as linking agents, bond the asphaltenes together, resulting in the asphaltenes clustering at the interface. This leads to the formation of a rigid film barrier with diminished interface activities at the w/o interface, making the increasing trend for the IFT at high salt concentrations.
Asphaltenes are considered “natural surface-active macromolecules” in crude oil that can reduce IFT between oil and water. However, their exact effect depends on concentration, asphaltene type, environmental conditions, and crude oil composition. So, depending on the concentration of salt and asphaltene, excessive asphaltene content can lead to aggregation and formation of rigid interfacial films. These aggregated structures increase interfacial viscosity and may increase interfacial tension rather than decrease it. Also, solid-like asphaltene networks can create mechanical resistance at the interface, making phase separation more difficult and leading to extremely stable emulsions.
For instance, in FW, the IFT values are 6.8, 7.9, and 12.1 mN/m for 0.2%, 4%, and 9% (w/v) asphaltene concentrations, respectively. Similar trends are observed for synthetic SW and other brine types such as SW2Ca, SW2Mg, SW0SO4, SW2SO4, SW4SO4, SW25d and SW50d, suggesting that increased asphaltene content results in higher IFT values. This implies that asphaltenes stabilize the oil/brine interface, which could influence the efficiency of oil recovery processes.
The coalescence time plot (Fig. 2f) reveals variations in the coalescence behavior of oil droplets across different brines. The coalescence time generally increases from FW to SW50d. Specifically, the coalescence time ranges from 29 s in FW to 52 s in SW50d, that related to the elasticity of the interface. This trend suggests that the brine composition (ion types) and concentration significantly impact the coalescence dynamics of oil droplets. Longer coalescence times in brines such as SW25d and SW50d indicate a more stable emulsion, which could hinder oil separation and recovery. In conclusion, the data demonstrate that asphaltene concentration and brine composition and concentration play crucial roles in determining the interfacial properties and coalescence behavior of oil/brine systems.
Higher asphaltene concentrations lead to increased IFT, more elasticity, stabilizing the oil/brine interface, while specific brine compositions like SW50d significantly prolong coalescence times, suggesting a more stable emulsion. These findings are important for optimizing enhanced oil recovery process, where the interplay between brine composition, heptol ratio and asphaltene content must be carefully managed to improve oil extraction effectively.
Our findings suggest that as the salinity decreases, the fluid–fluid interface exhibits enhanced elasticity. This heightened elasticity inhibits the snap-off of oil into smaller droplets dispersed in the brine. Consequently, in LSWF, the oil phase tends to be more continuous and mobile, making it easier to extract, in contrast to high salinity water flooding (HSWF). In addition, the ion concentration and type of ions also affect the elasticity, so that the removal of sulfate ions increases the coalescence time. Also, the amount of asphaltene and heptol ratio will be very effective on IFT and coalescence time. Therefore, for the design of LSW for injection and EOR, all these parameters must be considered simultaneously, and the result of their effect will be very effective in optimizing the design of the desired water.
Microscopy images of emulsions with diluted brine in heptol (Fig. 2e) show that emulsions exhibit more elastic interfaces in LSW. According to Fig. 2f, the efficiency of different individual water-soluble inorganic ions causes elasticity and coalescence time to decrease in the following order: FW, SW, SW2Mg, SW0SO4, SW2Ca, SW2SO4, SW4SO4, SW50d, and SW25d (for Asphaltene 0.2% (w/v) and heptol 1:5). All these suggest that the IFT and elasticity of the emulsion’s interfaces affected by decreasing the molarity of the salt, type of ions, heptol ratio and asphaltene concentration. These findings highlight the complexity of interfacial behavior in enhanced oil recovery and emphasize the need for precise control over injection water properties to optimize oil recovery. This complexity in determining interface properties for EOR has led us to develop a device that can simultaneously sense changes in wettability, which is discussed in Sections S5–S6 in the Supplementary Information, with statistical analysis presented in Figures S3-S4.
Based on these findings, it can be inferred that the poly dispersity index plays a significant role in destabilizing the interfacial film. This destabilization facilitates the coalescence of oil droplets, thereby promoting faster oil mobilization in water flooding processes. The Mg2+ and Ca2+ ions contribute to the formation of more elastic films than SO42− at the crude oil/brine interface, which are challenging to deform, consequently hindering the coalescence of oil droplets45. Emulsions characterized by high zeta potential (either negative or positive) are electrically stabilized, whereas emulsions with low zeta potentials tend to coagulate or flocculate, potentially leading to poor physical stability.
Water/rock interfaceZeta-potentials of water/rock interface
The inherent structure of carbonate mineral surfaces, including calcite and dolomite, comprises metal ions such as Ca2+ and Mg2+, coordinated to oxygen atoms from carbon atoms such as CO32−. Due to the reactive nature of the carbonate mineral surface, chemisorbed water molecules undergo dissociation, resulting in a surface characterized by hydroxylated cationic sites and protonated anionic sites. These sites, which are characterized by TPD methods, are stabilized by the presence of dissociated hydroxyl ions (OH-) and protons (H+), respectively47,48,49,50. It is anticipated that at low pH levels, the excess H+ ions and potential dissolution will lead to the dominance of positively charged cationic sites, rendering the overall surface positively charged. Conversely, at sufficiently high pH, excess OH- ions will induce a shift towards a more negative surface charge. However, the specific charge and stabilization of the surface site are contingent on both the brine composition and the pH. Under typical reservoir conditions, the carbonate rock surface, including calcite and dolomite, maintains a positive charge across the entire pH spectrum of the surrounding high-saline FW (Fig. 3a–c). This brine is characterized by a low concentration of negatively charged ions such as CO32− and SO42−, along with a substantial amount of positively charged Ca2+.
The observed rise in zeta potential with an increase in pH aligns with the theoretical framework of surface complexation modeling (SCM)5,6,26,27,51,52,53. According to SCM, the development of surface species, particularly > CaSO4− and, to a lesser extent, > CO3Ca+ and > CO3Mg+, significantly influences the overall surface charge. As pH rises, the negatively charged surface complex > CaSO4− undergoes substitution by the neutral > CaOH− moiety and > CaCO3, resulting in a reduction of the negative charge at the surface. The surface concentrations of > CO3Ca+ and > CO3Mg+ remain relatively constant despite pH changes. Surface complexation modeling suggests that, at higher salinities, the surface charge of carbonates becomes less responsive to pH fluctuations, leading to a smaller variation in zeta potential. The dilution of brine induces more negative surface charges due to increased concentrations of negatively charged species like > CO3−, a decrease in the overall concentration of positively charged complexes such as > CO3Mg+, and the formation of neutral moieties like > CaOH−. The statistical analysis of the data is detailed in Table S2 and Figures S5a, b in Sections S7–S8 in the Supplementary Information.
Total value of zeta-potentials for water/rock and oil/brine interfaces
The overall values of the zeta potential at various brine conditions, including both the oil/brine (Fig. 3ci-vi) and carbonate rock (calcite and dolomite)/brine interfaces, were assessed at different asphaltene concentrations and neutral pH (representative of reservoir conditions). The measured values are detailed and presented in Fig. 3c-v and c-vi.
The specific impact of sulfate content in synthetic SW was further investigated using SW and three modified brines (SW0SO4, SW2SO4, and SW4SO4), all maintaining nearly the same ionic strength as SW but varying in sulfate content from zero to four times that of SW. The findings reveal that the modified SW brine with four times the sulfate ions (SW4SO4) produce the most negative zeta potential, followed by SW2SO4, SW0SO4 and SW. This suggests that the presence of SO42− in the brine shifts the zeta potential to more negative values by increasing the population of sulfate anions in the EDL at the calcite surface. From an operational standpoint, reducing the SO42− concentration is preferable over increasing it due to the risks of reservoir souring and scaling associated with SW injection. Furthermore, calcite particles exhibit higher absolute zeta potential in SW4SO4, SW50d, SW25d, and SW2SO4 compared to SW and FW, and also compared to dolomite. In summary, both calcite and dolomite surfaces exhibit more negative zeta potentials with specific brine compositions such as SW2Ca, SW2Mg, SW0SO4, SW4SO4, and SW25d. The total zeta potential values are generally negative, with the most negative values observed in SW4SO4 and SW25d brines. These findings emphasize the importance of brine composition in determining the surface charge at oil/brine and rock/brine interfaces, which is crucial for optimizing EOR processes. Understanding these interactions can aid in selecting appropriate brine compositions to achieve desired interfacial behaviors under reservoir conditions. This phenomenon can also be analyzed in terms of surface chemistry. The results in Table 1 can be attributed to the increase in acidity and basicity sites for PDI adsorption on dolomite surfaces, which are higher (0.43 μmol per nm2) than on calcite crystals.
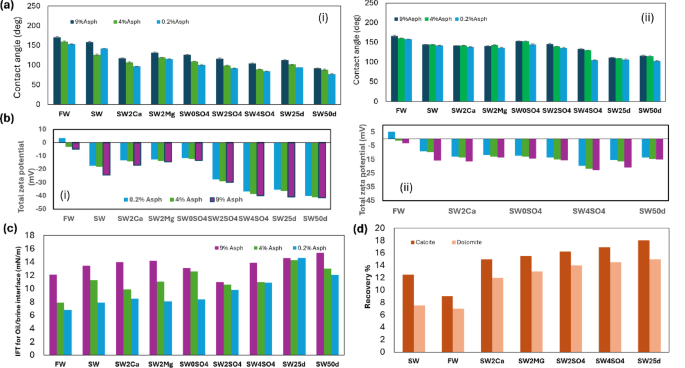
Wettability alteration (contact angle change) in water–rock interface
In SW, the contact angles for calcite are 143°, 129°, and 161° at asphaltene concentrations of 0.2%, 4%, and 9%(w/v), respectively (Fig. 4). For dolomite, the contact angles in SW are 143°, 146°, and 146° for the same asphaltene concentrations, suggesting a consistent trend toward increased oil-wettability with higher asphaltene concentrations. For SW25d, the contact angles for calcite are 94°, 103°, and 114° at asphaltene concentrations of 0.2%, 4%, and 9% (w/v), respectively, while for dolomite, they are 109°, 110°, and 112°. In SW0SO4, the contact angles for calcite are 103°, 110°, and 128° at asphaltene concentrations of 0.2%, 4%, and 9% (w/v), respectively, compared to 147°, 154°, and 155° for dolomite. In SW4SO4, the contact angles for calcite are 85°, 91° and 106° at asphaltene concentrations of 0.2%, 4%, and 9% (w/v), respectively, while for dolomite, they are 107°, 131°, and 136°. These results (Fig. 4a–h) highlight the significant impact of sulfate ions in modifying the surface wettability toward oil-wet conditions. Overall, these findings highlight the importance of both brine composition and asphaltene concentration in determining the wettability of rock surfaces, which in turn affects the efficiency of oil recovery processes. The varying contact angles across different brines and asphaltene concentrations highlight the need to tailor brine formulations to optimize wettability and enhance oil recovery. The contact angle observed in these experiments represents a macroscopic, effective contact angle, which is susceptible to factors such as contact angle hysteresis, surface roughness, and the preparation procedure. It is essential to emphasize that the key insight lies in the trend of contact angle changes after exposure to LSW rather than the initial contact angle value. The findings indicate that in FW, the oil droplet shapes tend to stabilize, reaching an equilibrium state where their contact angles remain constant. Upon transitioning to SW after 40 h, the contact angles for both oil droplets on calcite and dolomite decrease by 5° to 17°. From a statistical standpoint, the data analysis related to this section has been discussed in Section S9 of the Supplementary Information.
Contact angle of oil on rock in different brine and asphaltene content at (a) calcite and (b) at dolomite, brine interfaces and different, contact angle in degree (°) of oil (heptol 1:5 and Asph 0.2%) in SW25d against time on (c) calcite and (d) on dolomite rock, contact angle of total zeta potential distribution in both rocks (e) based on all brine types, (f) based on all stone types, images of contact angle of oil (heptol 1:5 and Asph 0.2%) in SW25d on (g) calcite and on (h) dolomite rock over 60 min (i)–(vi). (Scale bar = 3 mm).
Oil recovery in amott cell
Amott cell can be used to examine and compare the impact of various fluids on the wettability and absorption properties of rocks. For both calcite and dolomite, brines such as SW25d and SW4SO4 demonstrate the highest recovery values, reaching approximately 18 and 16.9 for calcite, and 15 and 14.5 for dolomite, respectively, by the end of the 72 h (Fig. 5a,b). In contrast, FW and SW show the lowest recovery rates, indicating their lesser effectiveness. These observations are consistent with the findings from the zeta potential measurements, which emphasize the important role of surface charge in influencing oil recovery. Higher total zeta potentials, as seen with SW25d and SW4SO4, are associated with greater oil recovery, suggesting improved wettability and stronger repulsion between oil droplets and rock surfaces. This enhanced recovery is attributed to the alteration of surface charges, which transition the wettability from oil-wet to more water-wet conditions, facilitating the release of oil attached to rock surfaces. Overall, the data highlights the importance of selecting appropriate brine compositions to optimize oil recovery processes in carbonate reservoirs.
The results suggest that the LSW and water with SO42− ions are likely influenced by alterations in surface charge and zeta potential behavior. The increase in the concentration ratio of SO42− to divalent cations appears to shift rock wettability toward a less-oil wetting state, consequently enhancing oil recovery in spontaneous imbibition5,7,11,54,55. The results highlight the significant influence of ions on altering the surface charge and wettability of the rock toward increased water-wetness, leading to a higher production of oil. The higher concentrations of Ca2+, Mg2+ and SO42− are associated with greater zeta potential, creating a more challenging environment for droplet coalescence due to electrostatic double layer repulsion.
Comparing three criteria zeta potential, IFT and contact angle for oil recovery
The performance of different brine types can be compared in terms of zeta potential, contact angle and IFT to determine the most effective brines for enhanced oil recovery (Fig. 6). The zeta potential analysis reveals that brines SW4SO4, SW25d and SW50d exhibit the most negative values, indicating higher stability. Lower contact angle values are preferable as they indicate more hydrophilic surfaces, which enhance oil recovery. In this regard, brines such as SW2Ca, SW2Mg, and SW2SO4 show lower contact angles, suggesting better performance for oil recovery. The IFT analysis indicates that SW50d, SW4SO4, and SW2Ca have higher IFT values, suggesting higher energy barriers for coalescence. Importantly, the zeta potential results align closely with both the IFT and contact angle findings. Brines that exhibit highly negative zeta potentials, such as SW4SO4 and SW25d, also tend to have high IFT values and suitable contact angles, indicating that these brines provide both stability and efficient oil recovery conditions. This alignment across multiple criteria reinforces the reliability of zeta potential as a comprehensive measure. In essence, zeta potential captures the electrostatic interactions at the oil-brine interface, which are fundamental to understanding and predicting the behavior of the system in terms of both IFT and wettability. Integrating these findings, SW4SO4, SW25d, and SW50d emerge as the most effective brines. SW4SO4 shows excellent performance across all criteria, with a highly negative zeta potential, high IFT values, and lower contact angles, making it particularly effective for enhancing oil recovery. Similarly, SW25d and SW50d also perform well, with highly negative zeta potentials and high IFT values, though their contact angles are slightly higher. Therefore, SW4SO4, SW25d, and SW50d are recommended as the best brines for achieving the desired outcomes in oil recovery processes for this reservoir, with SW4SO4 having a slight edge due to its lower contact angles. This comprehensive evaluation highlights the value of zeta potential as a measure that aligns well with other critical factors, ultimately guiding the selection of optimal brines for EOR.
Trend of (a) contact angles in (i) calcite, and in dolomite, (b) total zeta-potential in (i) calcite, and in dolomite; (c) IFT of A = FW, B = SW, C = SW2Ca, D = SW2Mg, E = SW0SO4, G = SW4SO4, H = 25dSW for 0.2% (w/v) asphaltene content for 02, 4 and 9% asphaltene content and (d) oil recovery for various brines of A = FW, B = SW, C = SW2Ca, D = SW2Mg, E = SW0SO4, G = SW4SO4, H = 25dSW for 0.2% (w/v) asphaltene content.
Zeta potential limitations and possible alternatives
Zeta potential measurements face some challenges, such as complex sample preparation, sensitivity to environmental conditions, and limited field applicability. In addition, their use is limited due to high equipment costs, time-intensive processes, and difficulties in high-salinity reservoirs. Furthermore, their indirect relationship to wettability and lack of real-time monitoring capabilities make them less practical for dynamic and heterogeneous oil recovery processes.
To address these challenges, charge sensors such as capacitive and field-effect transistor (FET) sensors56,57,58 present promising alternatives for charge sensing in oil recovery applications. These sensors enable real-time, in-situ monitoring of charge changes, allowing for rapid adjustments to reservoir conditions without requiring complex sample preparation. Capacitive sensors detect variations in capacitance caused by surface charge changes, while FET sensors measure shifts in surface potential, making them particularly suitable for high-salinity and dynamic environments. Their integration into advanced monitoring systems has the potential to overcome the limitations of zeta potential techniques, offering cost-effective and field-deployable solutions for enhanced oil recovery.
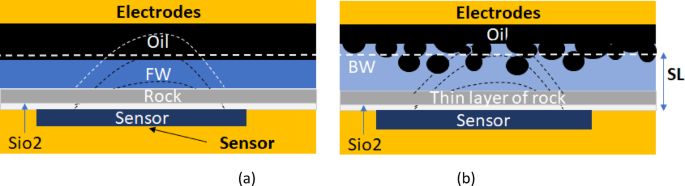
Despite their advantages, capacitive and FET sensors still face practical challenges. Developing sensing systems to select the optimal smart water for specific oil and rock combinations remains an ongoing effort. Additional difficulties include replicating the structure of reservoir rock as a thin layer above the sensor and beneath the formation water or smart water (as shown in Figs. 7a, b), ensuring the rock’s thin layer aligns with the sensor’s screen length, and reliably detecting charge variations under realistic reservoir conditions. Figure 7b illustrates the possibility of monitoring, in real-time, the removal or depletion of oil after injecting smart water, as the electrical field crosses the thin layers. Addressing these issues is crucial to fully harness the capabilities of capacitive and FET sensors in oil recovery applications.