The European preeclampsia market is set for robust expansion over the 2025-2035 decade, underpinned by rising maternal-health screening, growing adoption of biomarker diagnostics, and increasing commitment to early detection. As healthcare systems across the region strive to reduce pregnancy-related complications, preeclampsia diagnostics are emerging as a central component of prenatal care.
Market Size & Forecast
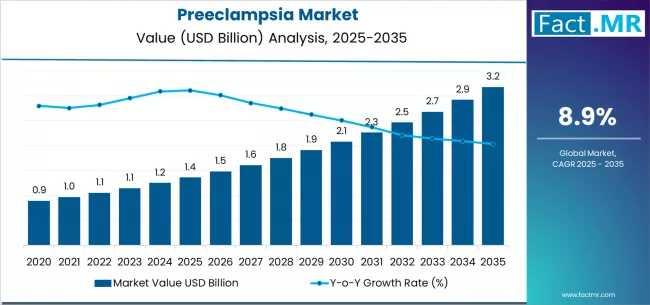
The global preeclampsia market is projected to expand from approximately USD 1.36 billion in 2025 to around USD 3.18 billion by 2035, equivalent to a compound annual growth rate (CAGR) of about 8.9%.
Europe is expected to mirror this strong growth trajectory. According to market analysis, the European preeclampsia diagnostics segment is already showing momentum with double-digit screening adoption, supported by clinical guidelines and advancing prenatal infrastructure.
To access the complete data tables and in-depth insights, request a Discount On The Report here: https://www.factmr.com/connectus/sample?flag=S&rep_id=11601
Key Drivers of Growth
Universal and Targeted Screening Uptake
Increasingly, European countries are integrating universal screening for preeclampsia into prenatal care routines, complemented by targeted testing for high-risk pregnancies. These combined approaches account for a large portion of diagnostic demand, as clinicians rely more on early risk stratification to guide patient monitoring.
Dominance of Biomarker-Based Blood Tests
Blood-based biomarker assays-particularly tests detecting placental growth factor (PlGF) and sFlt-1 (soluble fms-like tyrosine kinase-1)-are becoming standard. These biomarker tests are projected to capture a majority share of the diagnostics market, reflecting their clinical utility in predicting and diagnosing preeclampsia.
Technological Innovation & Point-of-Care Testing
Advances in microfluidic devices, multiplex immunoassays, and automated platforms are making preeclampsia testing faster, more accurate, and more accessible. These innovations are enabling diagnostic labs and prenatal clinics to deploy point-of-care solutions, allowing risk assessments earlier in pregnancy and in outpatient settings.
Policy Focus on Maternal Health
Maternal-health authorities and public health systems in Europe are increasingly prioritizing preeclampsia screening given its significant impact on maternal and fetal morbidity. Screening programs aligned with clinical guidelines are being expanded, backed by public health funding and modernization efforts in obstetric care.
Collaborative Clinical Care Models
Hospitals, diagnostic centers, and specialty clinics are forming integrated care models where high-risk pregnancies are managed in collaboration with obstetricians, maternal-fetal medicine specialists, and labs. This integrated approach is accelerating demand for consistent, repeatable, and high-quality diagnostic testing.
Browse Full Report https://www.factmr.com/report/preeclampsia-market
Challenges & Risks
Testing Costs & Reimbursement: High-performance biomarker assays can be expensive, and reimbursement schemes across different European countries may not yet fully cover advanced testing, limiting adoption in some markets.
Clinical Adoption Gap: Despite the clinical value of early detection, uptake of universal screening is uneven; some regions still rely on risk-based screening, which may miss preeclampsia cases.
Infrastructure Constraints: In smaller prenatal clinics or less-resourced regions, lack of equipment or trained personnel may hinder the implementation of sophisticated preeclampsia diagnostics.
Regulatory & Compliance Hurdles: Diagnostic companies must navigate varying regulatory pathways, quality standards, and data integration requirements across European markets.
Strategic Opportunities
Scaling Point-of-Care Diagnostics: Companies that can deliver low-cost, portable preeclampsia tests for use in community clinics or remote settings will be uniquely positioned to drive broad adoption.
Risk-Assessment Platforms: Developing integrated digital tools that combine biomarker results, clinical parameters, and patient history into risk-stratification platforms will help clinicians make informed decisions early in pregnancy.
Partnerships Across Healthcare Systems: Diagnostics firms can partner with national health services, maternal-fetal medicine departments, and lab networks to embed routine screening into standard prenatal workflows.
Next-Generation Biomarkers: Research into novel biomarkers beyond PlGF and sFlt-1-such as proteomic or metabolomic signatures-can open new avenues for more sensitive early warning tests.
Prenatal Health Education Campaigns: Building awareness among expectant mothers and prenatal care providers about the benefits of early preeclampsia screening can drive demand, especially for routine risk assessment.
Competitive Landscape
Major diagnostics and life-sciences companies are playing a pivotal role in Europe’s preeclampsia market by expanding their prenatal screening portfolios. These players are investing in advanced immunoassay platforms, predictive risk tools, and point-of-care devices. Partnerships between diagnostics firms, academic research centers, and maternal healthcare providers are also shaping the competitive environment, enabling faster translation of cutting-edge research into clinic-ready solutions.
Outlook & Conclusion
From 2025 to 2035, the European preeclampsia market is expected to grow strongly and sustainably, driven by rising clinical adoption, biomarker innovation, and an increasing policy emphasis on maternal health. With a projected CAGR close to 9%, the region represents a critical opportunity for diagnostics firms, healthcare providers, and prenatal care stakeholders.
Purchase Full Report for Detailed Insights
For access to full forecasts, regional breakouts, company share analysis, and emerging trend assessments, you can purchase the complete report here:
https://www.factmr.com/checkout/11601
Have a specific Requirements and Need Assistant on Report Pricing or Limited Budget please contact us – sales@factmr.com
To View Related Report :
Veterinary Antimicrobial Susceptibility Testing Market https://www.factmr.com/report/veterinary-antimicrobial-susceptibility-testing-market
Pain Management Drugs Market https://www.factmr.com/report/pain-management-drugs-market
Ventricular Assist Device (VAD) Market https://www.factmr.com/report/ventricular-assist-device-vad-market
Oral Mucositis Market https://www.factmr.com/report/oral-mucositis-market
– Contact Us –
11140 Rockville Pike, Suite 400, Rockville,
MD 20852, United States
Tel: +1 (628) 251-1583 | sales@factmr.com
About Fact.MR
Fact.MR is a global market research and consulting firm, trusted by Fortune 500 companies and emerging businesses for reliable insights and strategic intelligence. With a presence across the U.S., UK, India, and Dubai, we deliver data-driven research and tailored consulting solutions across 30+ industries and 1,000+ markets. Backed by deep expertise and advanced analytics, Fact.MR helps organizations uncover opportunities, reduce risks, and make informed decisions for sustainable growth.
This release was published on openPR.