Introduction
What are smart drugs?
Why multi-target approaches matter
Advantages
Challenges
Future outlook
References
Further reading
Smart multi-target drugs are reshaping modern therapeutics by addressing the complex, interconnected pathways that drive diseases like Alzheimer’s, cancer, and major depressive disorder. Advances in AI, molecular modeling, and network pharmacology are accelerating their development and improving therapeutic precision.
 Image Credit: Gorodenkoff / Shutterstock.com
Image Credit: Gorodenkoff / Shutterstock.com
Introduction
Traditional single-target drugs, while precise, are limited in their ability to address the complex biological networks underlying chronic diseases and prevent resistance. To overcome these issues and enhance treatment efficacy, smart drugs capable of modulating several pathways simultaneously offer a systems-level approach that aligns with the multifactorial nature of diseases such as Alzheimer’s, cancer, and major depressive disorder.7
What are smart drugs?
Smart or multi-target drugs are revolutionary therapeutic agents that concurrently regulate numerous receptors and signaling cascades. This multi-target drug design (MTDD) approach is rapidly reshaping modern pharmacology, powered by advances in artificial intelligence (AI), molecular modeling, and systems biology.1
AI-assisted molecular docking and virtual screening predict how compounds interact with various targets concurrently, while pharmacophore modeling identifies shared structural motifs that enable multi-receptor binding. Network pharmacology maps intricate relationships among drugs, targets, and disease circuits to identify synergistic interactions. Meanwhile, multi-objective optimization algorithms balance potency, selectivity, and pharmacokinetic properties, a workflow increasingly adopted in early-stage drug discovery pipelines.8
Through chemoinformatics and big data integration, researchers mine vast molecular and clinical datasets to identify novel scaffolds or ways in which existing drugs could be repurposed. Together, these tools are streamlining early drug discovery, reducing experimental failures by integrating omics data, patient-derived biomarkers, and AI-based predictive modeling.8,9
Why multi-target approaches matter
MTDDs overcome the limitations of single-target interventions by addressing the multifactorial and adaptive nature of many diseases. By coordinating effects across interlinked biological mechanisms, MTDD amplifies therapeutic benefit and reduces the likelihood of drug resistance, two major barriers in managing neurodegenerative and oncologic disorders.1,2
Alzheimer’s disease (AD)
Despite decades of research, anti-amyloid pharmacologic approaches have yielded limited benefit. New dual inhibitors that target glycogen synthase kinase-3 beta (GSK-3β) and tau, alongside β-site amyloid precursor protein cleaving enzyme 1 (BACE-1) modulators, show promise for slowing disease progression, especially given recent evidence highlighting the need to simultaneously modulate amyloid, tau, oxidative stress, and neuroinflammatory pathways.7
Compounds like deoxyvasicinone-donepezil hybrids and naturally derived cannabinoids including cannabidiolic acid (CBDA) and cannabigerolic acid (CBGA) also exhibit multi-target activity across cholinesterase and amyloid pathways; however, current evidence remains largely preclinical and in vitro, emphasizing the need for translational studies.3,7
Major depressive disorder (MDD)
MDD arises from disruptions in the interactions between serotonin and glutamate systems that govern mood, cognition, and neuroplasticity. Traditional selective serotonin reuptake inhibitors (SSRIs), which act on serotonin transporters, often yield delayed or partial responses. In contrast, advanced multimodal antidepressants regulate several receptor systems at once to restore neurochemical balance and optimize effectiveness.4
Vilazodone, which is currently approved for use in the United States, combines serotonin reuptake inhibition with partial 5-hydroxytryptamine receptor 1A (5-HT1A) stimulation to strengthen mood and cognitive function while limiting off-target side effects. Preclinical studies demonstrate that vilazodone produces greater serotonin release and stronger antidepressant-like responses than paroxetine or fluoxetine; however, higher doses may be associated with mild gastrointestinal effects. Vortioxetine acts on five serotonin receptor subtypes to exert antidepressant and pro-cognitive effects through indirect regulation of glutamate, an effect supported by evidence showing serotonergic modulation of AMPA and NMDA receptor-driven synaptic plasticity.4
Novel compounds like dextromethorphan-bupropion (Auvelity) and esketamine concurrently target N-methyl-D-aspartate (NMDA), monoamine, and brain-derived neurotrophic factor (BDNF)-linked neuroplasticity. The U.S. Food and Drug Administration (FDA)-authorized esketamine nasal spray offers rapid relief in recalcitrant depression, though heterogeneity in Phase III trial results underscores the need for biomarker-driven patient selection.5
Cancer
In oncology, relapse remains a major concern, as it contributes to nearly 90% of cancer-related deaths. Multi-action compounds block compensatory signaling to enhance response rates, limit tolerance, and ultimately improve patient outcomes.
For example, dual-pathway inhibitors targeting phosphatidylinositol 3-kinase/mammalian target of rapamycin (PI3K/mTOR) and rapidly accelerated fibrosarcoma/mitogen-activated protein kinase (RAF/MEK) prevent pathway reactivation, which leads to sustained therapeutic responses.6
Novel drugs such as imatinib and sunitinib pioneered this approach by simultaneously inhibiting multiple tyrosine kinases, including breakpoint cluster region-abelson (BCR-ABL), the c-KIT proto-oncogene, and platelet-derived growth factor receptor alpha (PDGFR). These drugs have transformed outcomes in chronic myeloid leukemia (CML), gastrointestinal stromal tumors (GIST), and renal cancers. Second-generation inhibitors, including pazopanib, cabozantinib, and entrectinib, further increase precision and blood-brain barrier (BBB) penetration, thereby prolonging survival, underscoring the benefit of targeting multiple nodes within oncogenic signaling networks.6
 Image Credit: Gorodenkoff / Shutterstock.com
Image Credit: Gorodenkoff / Shutterstock.com
Advantages
Smart network-based drugs offer a transformative approach for treating biologically intricate diseases by providing broader molecular coverage and pathway-level synergy within a single molecule. Unlike single-target agents, these compounds can modulate several interdependent biological mechanisms simultaneously, producing additive or synergistic effects that strengthen overall clinical performance. This concerted activity allows lower dosage requirements, thereby minimizing toxicity while maintaining potency.6,7
In AD, MTDD enhances BBB penetration, reduces metabolism-related toxicity, and limits polypharmacy. Innovative proteolysis-targeting chimeras (PROTAC)-based designs employing triazole ligation chemistry are associated with selective degradation of pathogenic tau proteins with greater BBB permeability, though current PROTAC candidates remain in preclinical development.7
Similarly, ribonucleic acid (RNA)-based therapeutics such as n-acetyl-D-galactosamine (GalNAc)-conjugated small interfering RNA (siRNA) and stable nucleic acid lipid particle (SNALP) delivery systems have achieved efficient brain delivery and robust silencing of disease-relevant transcripts in animal models, but clinical translation remains limited.7
Beyond neurodegeneration, multi-target-directed ligands (MTDLs) in oncology and infectious diseases reduce drug tolerance by targeting multiple nodes within adaptive signaling networks. Collectively, these strategies broaden the therapeutic window while enhancing patient compliance, safety, and the durability of treatment responses.6
Challenges
Despite their potential to transform therapy, smart integrated drug designs present significant scientific, technical, and regulatory challenges. Designing a single molecule capable of modulating diverse networks requires an extensive understanding of disease network biology and inter-target interactions.
Balancing efficacy across several targets without inducing off-target toxicity remains a significant challenge. Multi-target compounds may unintentionally interfere with unrelated biological molecules, leading to adverse effects or unpredictable pharmacological outcomes.6
Optimizing absorption, distribution, metabolism, and excretion (ADME) profiles for drugs that act on different sites increases the complexity of designing these smart drugs, especially because physicochemical properties suitable for one target class may hinder interactions with another.2
Multi-target agents also require more extensive preclinical validation, integrated pharmacokinetic studies, and longer clinical trials to assess safety and systemic interactions. Taken together, these factors lead to higher research and development (R&D) costs and attrition rates due to long timelines, high failure rates, and the technical difficulties of acting across coordinated molecular processes.7,8
In neurodegenerative diseases like AD, crossing the BBB is a significant limitation that is further complicated by heterogeneity in patient pathology, necessitating individualized target prioritization strategies.7 Nevertheless, recent advances in AI-driven modeling, nanocarrier systems, and rational target prioritization have the potential to overcome these challenges.7
Future outlook
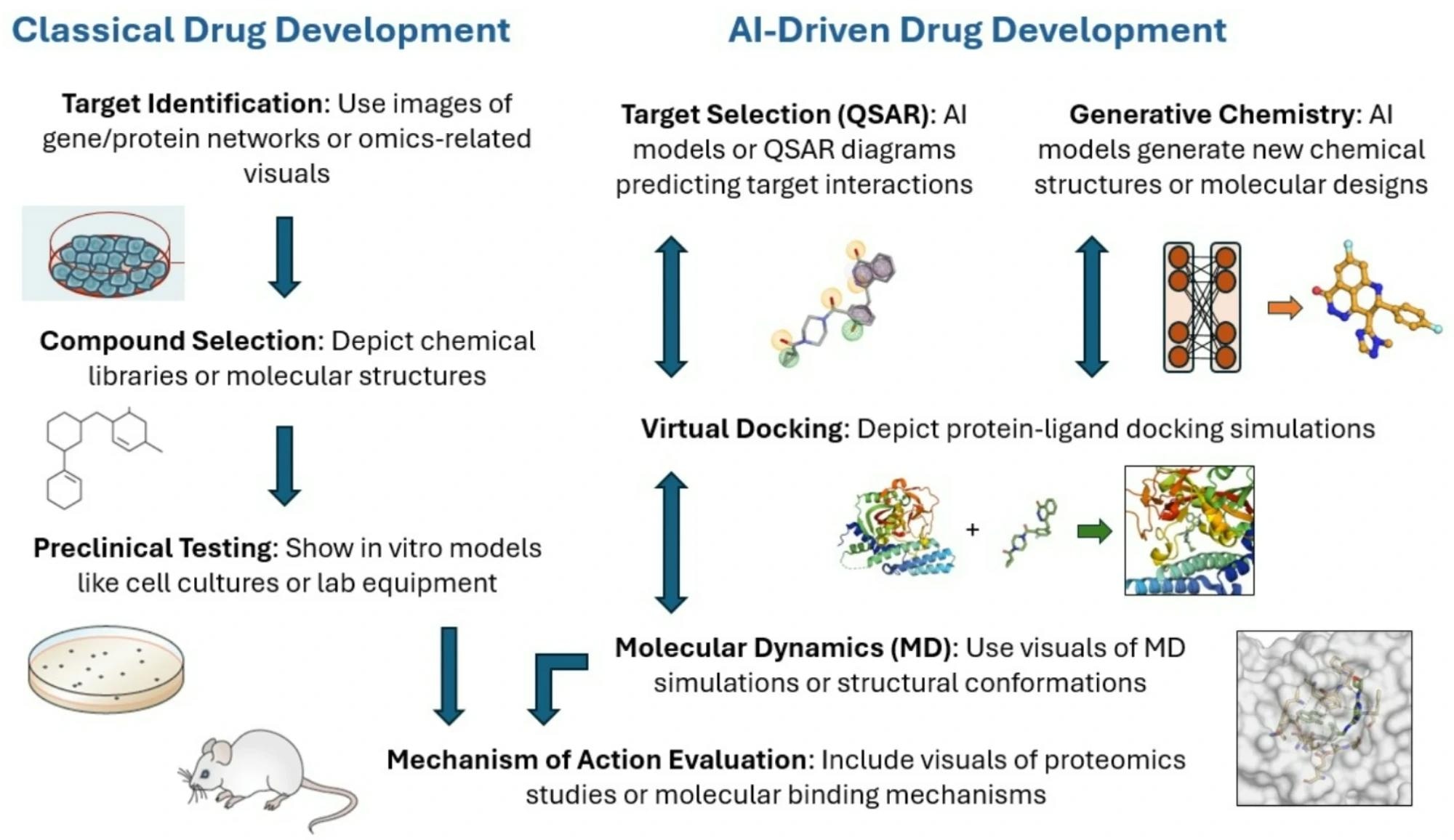
Advances in AI-driven drug design and digital biomarker integration are accelerating the development of smart multi-target drugs. AI can analyze vast biological datasets to identify synergistic targets, optimize lead compounds, and predict clinical efficacy and safety, all of which reduce development timelines and trial failures by enabling real-time simulation of biological responses and digital twin–based trial optimization.8,9
Description of classical versus AI-driven drug development
Simultaneously, digital biomarkers from wearables and mobile sensors facilitate continuous, real-world monitoring of treatment responses, supporting personalized therapy strategies. Together, these innovations are reshaping drug discovery into a data-informed and patient-centered process.8,9
References
Yang, T., Sui, X., Yu, B., et al. (2020). Recent Advances in the Rational Drug Design Based on Multi-target Ligands. Current Medical Chemistry 27(28);4720–4740, DOI:10.2174/0929867327666200102120652, https://www.benthamscience.com/article/103413.
Zięba, A., Stępnicki, P., Matosiuk, D., & Kaczor, A. A. (2022). What are the challenges with multi-targeted drug design for complex diseases? Expert Opinion on Drug Discovery 17(7);673–683. DOI:10.1080/17460441.2022.2072827, https://www.tandfonline.com/doi/full/10.1080/17460441.2022.2072827.
Prati, F., Bottegoni, G., Bolognesi, M. L., & Cavalli, A. (2018). BACE-1 Inhibitors: From Recent Single-Target Molecules to Multi-target Compounds for Alzheimer’s Disease. Journal of Medicinal Chemistry 61(3);619–637. DOI:10.1021/acs.jmedchem.7b00393, https://pubs.acs.org/doi/10.1021/acs.jmedchem.7b00393.
Pehrson, A. L., & Sanchez, C. (2013). Serotonergic modulation of glutamate neurotransmission as a strategy for treating depression and cognitive dysfunction. CNS Spectrums 19(2);121. DOI:10.1017/S1092852913000540, https://www.cambridge.org/core/journals/cns-spectrums/article/serotonergic-modulation-of-glutamate-neurotransmission-as-a-strategy-for-treating-depression-and-cognitive-dysfunction/2B2E78EEE315FADE0FF877076D35A0B9.
Vecera, C. M., Jones, G., & Soares, J. C. (2023). Pharmacotherapies Targeting GABA-Glutamate Neurotransmission for Treatment-Resistant Depression. Pharmaceuticals 16(11);1572. DOI:10.3390/ph16111572, https://www.mdpi.com/1424-8247/16/11/1572.
Doostmohammadi, A., Jooya, H., Ghorbanian, K., et al. (2024). Potentials and future perspectives of multi-target drugs in cancer treatment: the next generation anti-cancer agents. Cell Communication and Signaling 22(228). DOI:10.1186/s12964-024-01607-9, https://biosignaling.biomedcentral.com/articles/10.1186/s12964-024-01607-9.
Hossain, M. S., & Hussain, M. H. (2025). Multi-Target Drug Design in Alzheimer’s Disease Treatment: Emerging Technologies, Advantages, Challenges, and Limitations. Pharmacology Research & Perspectives 13(4);e70131. DOI:10.1002/prp2.70131, https://bpspubs.onlinelibrary.wiley.com/doi/10.1002/prp2.70131.
Ocana, A., Pandiella, A., Privat, C., et al. (2025). Integrating artificial intelligence in drug discovery and early drug development: a transformative approach. Biomarker Research 13(45). DOI:10.1186/s40364-025-00758-2, https://biomarkerres.biomedcentral.com/articles/10.1186/s40364-025-00758-2.
Bhushan, A., & Misra, P. (2025). Unlocking the potential: multimodal AI in biotechnology and digital medicine – economic impact and ethical challenges. npj Digital Medicine 8(619). DOI:10.1038/s41746-025-01992-6, https://www.nature.com/articles/s41746-025-01992-6.
Further Reading
Last Updated: Nov 25, 2025