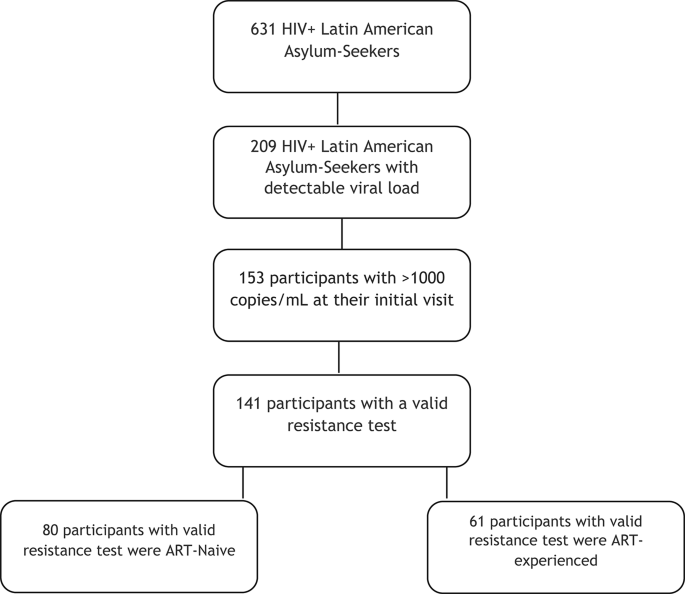
Participants
Between January 2022 and June 2023, 631 Latin American asylum seekers living with HIV were evaluated for the first time at our institution after they arrived in Spain. Of these, 209 had a positive viral load at their initial evaluation, with 153 presenting plasma VL > 1,000 copies/mL. Genotypic resistance testing was performed in all these participants. Of these, 12 tests failed due to poor RNA quality—most often related to delayed sample processing or degradation—and, in some cases, to primer mismatch in non-B HIV subtypes. The remaining 141 cases yielded valid resistance results and were included in the analysis (Fig. 1). Participants were stratified into two groups according to their treatment history: those with prior ART exposure (n = 68) and ART-naïve individuals (n = 85).
Flowchart of individuals included in the study.
The mean age of the participants was 32.4 (SD = 6.6) years. Most identified as cisgender men (127; 83%), followed by transgender women (19; 12.4%) and cisgender women (7; 4.6%). The most frequent countries of origin were Colombia (51; 33.3%), Venezuela (40; 26.1%), and Peru (23; 15%). Comprehensive baseline characteristics can be found in Table 1.
Table 1 Characteristics of Latin American asylum seekers with HIV and viral load >1,000 copies/mL at their first clinical visit upon arrival in Spain, January 2022−June 2023.
At their initial clinic visit, 71.9% of participants reported difficulties accessing the healthcare system, with a significantly higher proportion among previously treated individuals compared to ART-naïve individuals (82.3% vs. 63.5%, p = 0.024). The median time since HIV diagnosis was 3 years (IQR: 1–7), with notable differences by treatment history: 2 years (IQR: 1–2) among ART-naïve individuals and 7 years (IQR: 4–9) among those with previous ART exposure. Among the 68 participants with prior ART, only 33 (48.5%) were on treatment upon arrival in Spain, and just 7 (10.2%) were still on therapy by their first clinic visit. Consequently, 61 (89.7%) were not receiving any medication at that evaluation. The median duration of prior ART was 6.7 months (IQR: 3.4–48.1), whereas the median time off treatment was 8 weeks (IQR: 4.0–23.5).
Previous ART regimens
Among the 68 patients who had received prior antiretroviral therapy (ART), non-nucleoside reverse transcriptase inhibitor (NNRTI)–based regimens were the most common (48.6%), followed by integrase strand transfer inhibitor (INSTI)–based regimens (27.9%) and protease inhibitor (PI)–based regimens (14.7%). In 8.8% of cases, the specific regimen was unknown due to incomplete recall. A detailed breakdown of these regimens, including specific combinations and percentages, is provided in Supplementary Table 2. It is important to note that these reflect the regimens reported at arrival, although other treatments may have been used previously.
Resistance patterns
Of the 153 individuals with a viral load > 1,000 copies/mL, 12 (7.8%) could not undergo antiretroviral resistance testing due to sequencing inhibition, leaving 141 participants eligible for resistance analysis. Among them, 32 (22.7%) exhibited at least one surveillance drug resistance mutation (SDRM) as defined by the WHO (NNRTI, NRTI, or PI), with a higher prevalence in previously treated individuals (32.8%) compared to ART-naïve patients (15%), as shown in Table 2.
Table 2 Prevalence of surveillance drug resistance mutations (SDRM) for reverse transcriptase inhibitors and PIs among study participants (n = 141).-NRTI and NNRTI mutations
Overall, 7.1% (10 participants) of the cohort harbored NRTI mutations. The prevalence was significantly higher in the previously treated group (13.1%) compared to the naïve group (2.5%, p = 0.015). Among the NRTI class, it is worth mentioning that 11.4% of previously treated patients demonstrated resistance to 3TC/FTC, whereas no resistance was observed in the naïve group (Fig. 2).
Percentage of Resistance to NRTIs drugs Expressed by Type of Antiretroviral Treatment and Corresponding Subgroup (n = 141). ABC, abacavir; AZT, zidovudine; 3TC, lamivudine; FTC, emtricitabine; TDF, tenofovir disoproxil fumarate.
NNRTI mutations were found in 30 participants (21.3%), affecting 13.8% of the ART-naïve group and 31.1% of those with prior treatment (p = 0.012). Within the NNRTI class, EFV and NVP resistance rates were notably higher in the previously treated group, at 31.1% and 32.7%, respectively, compared to 15.1% resistance for both EFV/NVP in the naïve group. Similarly, RPV showed a higher resistance rate in patients with prior ART exposure (19.7%) versus those naïve to treatment (5.1%, Fig. 3).
Percentage of Resistance to NNRTIs drugs Expressed by Type of Antiretroviral Treatment and Corresponding Subgroup (n = 141).
Further details—including low, moderate, and high resistance levels per the Stanford criteria—can be found in Supplementary Table 2, where “resistant” encompasses any degree of reduced susceptibility.
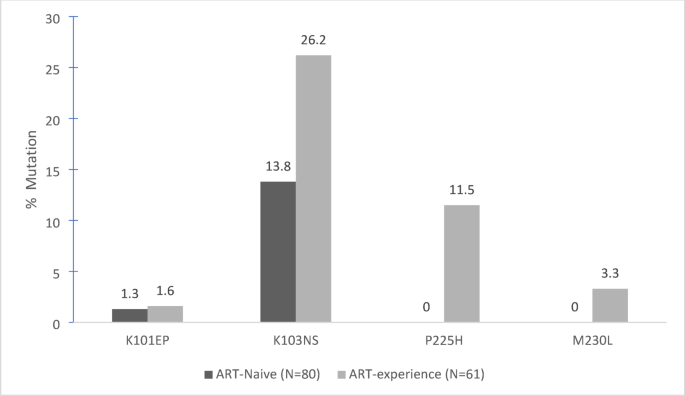
Supplementary Table 3 presents all identified SDRMs, categorized by NNRTIs, NRTIs, and subgroups, respectively. In Figs. 4 and 5, the most frequent NNRTI and NRTI SDRMs are detailed. Of note, the K103NS mutation was found in 13.8% of naïve patients and 26.2% of those with prior treatment. Regarding NRTIs, M184V emerged in approximately 10% of the previously treated cohort and was not detected among naïve participants.
Most Frequent SDRM for NNRTIs in Previously Treated and Naive Patients (n = 141).
Most Frequent SDRM for NRTIs in Previously Treated and Naive Patients (n = 141).
-PI mutations
Protease inhibitor mutations were detected in 7 patients (5%), with a slightly higher rate among naïve individuals (6.3%) compared to those previously treated (3.3%, p not statistically significant). The specific mutations—Q58E (3 cases), M46ML (1 case), L33F (1 case), and L10LF/L10F (2 cases)—did not confer clinically significant PI resistance, indicating that these variations are less likely to compromise PI-based therapy.
Factors associated with resistance
Both univariate and multivariable logistic regression analyses were conducted to identify factors associated with clinically significant resistance. Based on clinical relevance, we retained two predictors in the final multivariable model: HIV infection duration > 5 years and prior ART exposure. Age and sex were also included as covariates. In the adjusted model, HIV infection duration > 5 years was independently associated with the presence of SDRMs (adjusted OR:3.82 (95% CI: 1.084–13.512, p = 0.037), while prior ART use did not reach statistical significance. No significant associations were observed for age or sex (Table 3).
Table 3 Multivariate logistic regression analysis of variables associated with clinically significant resistance or SDRM* (n = 141).