A substantial amount of research has confirmed that the formation of diamondoids is closely related to the thermal maturation of organic matter. Fang et al.34 proposed that the evolution of diamondoids in petroleum was divided into three main stages, i.e., early generation stage (Ro at 0.8% − 1.0%), high-temperature cracking stage (Ro > 1.0%), and cracking stage. Wei et al.5 suggested that diamondoid compounds underwent stages of generation, enrichment, and destruction with increasing thermal maturity, where they suggested that the generation stage was at Ro 4.0%. Although there are some differences in the criteria for dividing the evolutionary stages in these studies, it is evident that thermal maturity plays a decisive role in the formation and destruction of diamondoids.
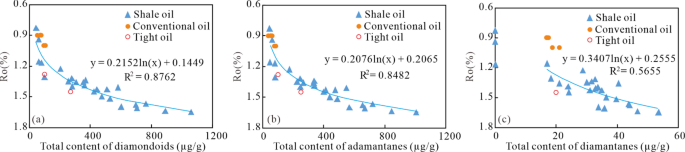
The Gulong shale oil is characterized by in-situ accumulation19,22, which means that the burial depth of shale or the thermal maturity of organic matter controls the content and distribution characteristics of diamondoids in the shale oil. As shown in Fig. 6, the total diamondoid content and adamantane content are closely related to Ro, with correlation coefficients R2 greater than 0.84, indicating that the formation of diamondoids and adamantanes is mainly controlled by thermal maturity. The relationship between the content of diamantanes and Ro is weaker, with a correlation coefficient R2 greater than 0.56, suggesting that the content of diamantanes is not only affected by thermal maturity, but also by other factors.
According to the relationship between diamondoid content and Ro shown in Fig. 6, four stages can be distinguished. (1) Immature to low-mature stage with Ro 1.4%; diamondoid content increases rapidly. The total diamondoid content ranges from 300 to 1000 µg/g, adamantane content ranges from 280 to 1000 µg/g, and diamantanes are present with content around 25 to 55 µg/g. This indicates significant rearrangement and cracking reactions in the shale oil.
Relationship between diamondoid content and clay mineral catalysis
Multiple studies have confirmed that diamondoid compounds can be formed from polycyclic hydrocarbons under high-temperature conditions, catalyzed by Lewis acid in clay minerals6,15,35,36. Smectite and other clay minerals play an important role in the formation of diamondoids. Chao37 demonstrated through pyrolysis experiments that the Lewis acidity decreases gradually during the transformation of smectite to illite. Research on the diagenetic evolution of clay minerals in the Gulong shale shows that in the stage with Ro 38. After Ro > 1.0%, clay minerals are dominated by illite-smectite mixed layers and illite, with the mixed layers progressively transforming into illite with increasing thermal maturity. Although the catalytic activity of clay minerals is strong in the early stages of diagenesis and weakens in later stages, their catalytic role remains significant as organic matter begins to undergo rearrangement and cracking reactions37.
At a similar higher thermal mature stage, tight oil often contains a higher concentration of diamondoids than the shale oil (Fig. 6). For example, in the Qijia-Gulong Sag, the tight oil from Well HT1H in the Fuyu oil layer has a Ro of approximately 1.45%, with a total diamondoid content of 270 µg/g, adamantane content of 251 µg/g, and diamantane content of 19 µg/g. In contrast, the shale oil from the adjacent Well GY8HC, which is sourced from the overlying source rock, has a total diamondoid content of 362 µg/g, adamantane content of 339 µg/g, and diamantane content of 24 µg/g. Since tight oil originates from early-generated crude oil in the overlying Qingshankou Formation shale18, it has experienced higher temperatures than its overlying source rock after entering the reservoir. However, the content of all types of diamondoids in shale oil is 1.26 to 1.35 times higher than that in tight oil. This indicates that under the same thermal evolution conditions, the cracking degree of crude oil in non-clay reservoirs, of which the clay mineral content is between 4.7% − 13.8%, is significantly lower than that in clay-rich shale, whose clay mineral content is 32.4% − 56.1%. This suggests that the catalytic action of clay minerals in shale promotes the cracking of shale oil and the formation of diamondoids. In contrast, tight sandstone reservoirs, which contain fewer clay minerals and have weaker catalytic activity, have lower diamondoid content.
Relationship between diamondoid content and overpressure in oil reservoirs
Song et al.16 argued that under overpressure conditions, the acidic catalytic activity of clay minerals was enhanced. Under high-temperature and high-pressure conditions, the acidic sites on the surface of clay minerals can adsorb and activate lower-grade diamondoids with alkyl substitution in crude oil, making them more susceptible to rearrangement and homologation reactions, thereby generating higher-grade diamondoids. Additionally, the overpressure state increases the intensity of molecular motion in crude oil, leading to more frequent collisions between molecules and accelerating the rate of diamondoid formation. Lin and Wilk6 also proposed a similar argument, suggesting that higher grade diamondoids in crude oils were generated from lower grade diamondoids with alkyl substitution through homologation reactions under higher subsurface temperatures and pressures.
In this case, comparing the diamondoid content of shale oil with similar maturity but different pressure environments during the high-maturity stage also suggests the impact of overpressure on diamondoid formation. For example, a shale oil (Ro 1.31%) from Well SYY1 in the Qijia-Gulong Sag, located 20 m horizontally from a fault, has a lower reservoir pressure coefficient (1.3) due to the oil expulsion through the fault; the total diamondoid content is 119 µg/g, adamantane content is 119 µg/g, and diamantanes are absent. In contrast, shale oil from Well GY7 in the area has close thermal maturity (Ro 1.35%), but is far away from the fault, with horizontal distance at 1004 m; it shows no significant vertical hydrocarbon expulsion, and the reservoir pressure coefficient is 1.5. The shale oil from this well has a total diamondoid content of 281 µg/g, adamantane content of 247 µg/g, and diamantane content of 7 µg/g. The total diamondoid content and adamantane content in the Well GY7 are 2.4 and 1.9 times those in the Well SYY1, respectively; particularly, the presence of diamantane in Well GY7 strongly suggests that overpressure significantly enhances the formation of higher-grade diamondoids. Measured reservoir pressure coefficients in the Gulong shale indicate that they are closely related to the thermal maturity. The pressure coefficient is 1.0 for Ro 1.2%. Therefore, it can be expected that overpressure exerts a more significant influence on diamondoid formation when Ro exceeds 1.2%. Notably, the correlation between diamantane content and maturity in shale oil (Fig. 6) is weaker than that observed for adamantanes, implying that the diamantane content may be more influenced by reservoir pressure. In shale oils with close thermal maturity from different areas, variations in through-going faults and formation pressure coefficients lead to significant differences in diamantane content. The reason may be that the increase in pressure changes the structure of clay minerals and enhances their catalytic activity39.
Relationship between diamondoid composition and crude oil maturity
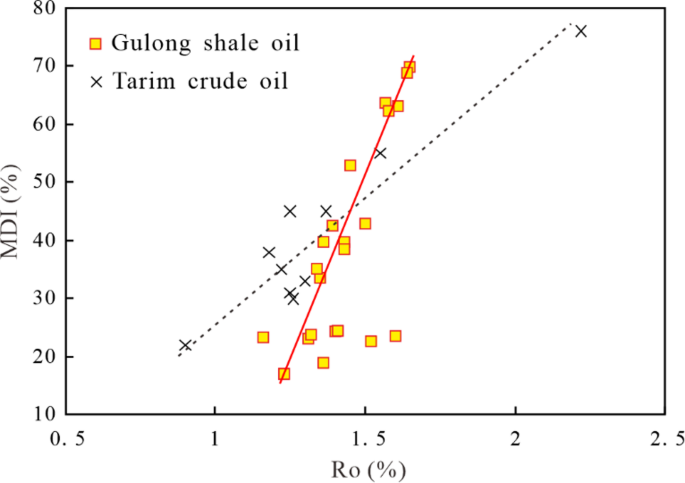
The compositional distribution of diamondoids in shale oil is closely related to their thermal maturity. Chen13 established a relationship between Ro and MDI (Methyl Diamantane Index: 4-MD/ (1-MD + 3-MD + 4-MD)) for conventional oil in the Tarim Basin; as shown in Fig. 7, the Ro ranges from 0.9% to 2.2%, while MDI ranges from 20% to 75%. In contrast, as for the Gulong shale oil, Ro ranges from 1.1% to 1.6%, while the MDI mainly ranges between 15% and 70% (Fig. 7). Compared to the conventional oil in the Tarim Basin, the rapid increase of the MDI with increasing thermal maturity may be attributed to the enhanced clay mineral catalytic effect under high-temperature and high-pressure conditions present in Gulong shale oil reservoirs, as discussed before.
Relationship between Ro and MDI in the Tarim crude oil13 and Gulong shale oil.
DAHL et al.1 proposed a calculation formula for evaluating the degree of oil cracking using the absolute content of 4-MD + 3-MD:
$$\:{C}_{R}=\left(1-\frac{{C}_{0}}{{C}_{c}}\right)\times\:100\%$$
Where: C0 is the concentration of (4-+3-) MD in the uncracked sample (µg/g), which is the baseline value of (4-+3-) MD; Cc is the concentration of (4-+3-) MD in samples of any maturity (µg/g); CR represents the degree of oil cracking.
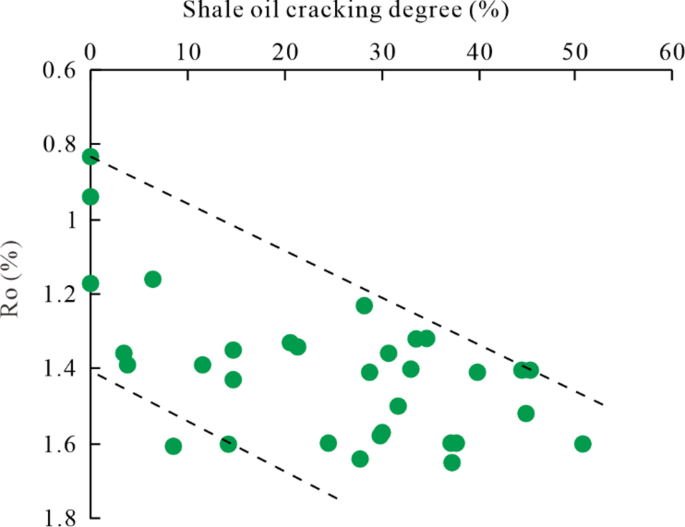
The basis for evaluation lies in determining the baseline value of 4-MD + 3-MD in a basin. In this study, the baseline value of (4-+3-) MD of conventional oil, which is 4.6 µg/g, is used as the reference. This value is comparable to the global baseline level of 4 to 5 µg/g for crude oil. For the Gulong shale oil, the concentration of 4-MD + 3-MD is generally in the range of 4 to 9 µg/g. The cracking degree of the Gulong shale oil is estimated to be between 0% and 50% (Fig. 8). Specifically, for the shale oil with Ro between 1.2% and 1.4%, the cracking degree ranges from 0% to 35%, while for the shale oil with Ro > 1.4%, the cracking degree ranges from 8 to 51%.
As shown in Fig. 8, shale oil samples with close maturity show a significant difference in the cracking degree, implying that the shale oil cracking may be impacted by shale reservoir fluid pressure and clay mineral content, as discussed above. As discussed in 4.3, shale oil reservoirs close to a fault tend to have less fluid pressure; as a result, corresponding shale oils may have less diamondoids and thus oil cracking degree.
Notably, in contrast to hydrocarbon reservoirs in other basins, which may have experienced migration or secondary alteration, the Gulong shale oil is characterized by in-situ accumulation. The oil has not undergone large-scale migration, gas washing, or biodegradation, and its high diamondoid content indicates the characteristics of in-situ cracking and rearrangement to form diamondoids. In contrast, conventional oils in the Daqing Placanticline have relatively low total diamondoid content, but the content of diamantanes is significantly higher than that in shale oil with the same maturity (Table 3), indicating that the diamondoid content is mainly contributed by early oil generation, with some diamantanes originating from late-stage oil generation.
Formation and evolution pattern of diamondoids
Based on the analysis of diamondoid formation processes and the relationship between their relative abundance and key controlling factors, the formation of diamondoids in the Gulong shale oil is divided into four distinct stages (Table 5).
Primary stage (Ro 40,41,42. The diamondoids are mainly adamantanes, and the content is generally less than 60 µg/g.
Inheritance stage (Ro = 0.8%−1.2%): The thermal degradation of organic matter is enhanced; as a result, diamondoids increase slightly. However, diamondoids remain predominantly adamantanes, with the content less than 100 µg/g.
Generation stage (Ro = 1.2%−1.4%): Oil starts to crack, with the maximum cracking degree reaching up to 35%. The cracking of shale oil positively impacts the formation of diamondoids. Additionally, the catalytic effect of clay minerals combined with overpressure accelerates the formation of diamondoids. This is characterized by a marked increase in the quantity of adamantanes, with the highest content reaching up to 300 µg/g. Diamantanes also begin to appear, but are present in small amounts, with the content less than 25 µg/g.
Enrichment stage (Ro > 1.4%): Oil undergoes significant thermal cracking at this stage, indicated by a rapid rise in diamondoids. For example, the shale oil in the Qijia-Gulong Sag shows a maximum cracking degree of 51% and a minimum density of 0.77 g/cm³ (Table 1). Additionally, the catalytic effect of clay minerals can increase diamondoids by 1.3 times, while overpressure in reservoirs can further boost diamondoids by approximately 2 times. This results in adamantane compounds reaching a maximum content of up to 1000 µg/g and diamantanes reaching a maximum of up to 55 µg/g. The combined influence of these factors promotes the enrichment of diamondoids.