The hypothesis that both subcutaneously applied methadone and transdermal fentanyl solution can provide adequate postoperative analgesia in dogs after thoracolumbar neurosurgery could be confirmed. All three used pain scales provided reasonable monitoring of the animals. In contrast, von Frey Filament testing did not appear helpful for the clinical evaluation of pain in these dogs after hemilaminectomy.
Owing to the low number of animals requiring rescue analgesia or showing undesirable effects, both analgesic courses (SC methadone and transdermal fentanyl) appear clinically comparable and adequate. Both drugs are µ-, κ- and δ- opioid receptor agonists [20, 21]. They are therefore classified as effective analgesics, which explains their effectiveness. However, based on in vitro studies, for both methadone isomers dose-dependent binding and antagonism at the NMDA receptor are being discussed [22, 23], which theoretically could be beneficial in dogs with spinal disease. Intervertebral disc disease often involves neuropathic pain components, which could benefit from drugs that influence NMDA receptor activity [24, 25]. An antihyperalgesic effect of the D- isomer of methadone has been documented in laboratory rodents [26]. However, in the studied clinical cases, no benefit of the possible NMDA action was detectable. Independent of the treatment group all dogs in the current trail received the same dose of levomethadone as part of anaesthesia premedication. It is possible that this still could have an analgesic effect at the first post operative measurement points and therefore could have blunted existing group differences during the early postoperative time. As no plasma levels of methadone and metabolites were measured this effect cannot be excluded.
Furthermore, the application of pregabalin to all dogs could have attenuated smaller differences between treatment groups M and F. Including pregabalin in the postoperative analgesic plan for dogs with disc herniation led to favourable analgesia, as shown by reduced pain scores compared with methadone alone [18]. However, only trends toward lower pain scores are documented when gabapentin is used in a similar setting [27]. Additionally, in humans, pregabalin is known to be effective in patients suffering from various diseases leading to neuropathic pain [28, 29]. Furthermore, opioid-sparing effects of pregabalin have been documented in humans and laboratory rodents [30, 31]. At present, no data concerning pregabalin opioid sparing in dogs exist. But plasma levels in dogs documented after 4 mg/kg orally applied pregabalin as a single dose (4.1 µg/ml) or after repeated dosing every 8 h (5.1 µg/ml) are in the range for which analgesia in humans is expected [18, 32].
The subcutaneous route for methadone application was chosen. At present, most postoperative analgesic plans, including methadone use intravenous drug administration [33, 34]. The literature concerning the subcutaneous use of methadone in dogs is limited [10, 12, 35]. The dosing regimens and durations of treatment used in these studies are diverse. Pharmacokinetic data in dogs, indicating a longer half-life of methadone when it was injected subcutaneously (10.7 ± 4.5 h) compared to when it was applied intravenously (3.9 ± 1.0 h) [9]. Further plasma concentrations 6 h after SC methadone application (18–28 ng/ml) are known to be just above the level causing thermal and mechanical antinociception in beagle dogs (17 ng/ml) [10]. On that basis a treatment of four times daily was scheduled [9, 10] for the current trial. Focussing solely on the documented mean half-life of methadone after subcutaneous injection, dosing only once or twice daily seems to be possible. However, owing to the rather large variability in half-life times, the data available about plasma levels and the lack of preexisting clinical data, more frequent dosing (four times daily) was performed. Judging the low need for rescue analgesia in the present study, this dosing regimen seems to be adequate for dogs after neurosurgery. However, four dogs in group M showed dysphoric behaviour, which resolved without medical intervention and did not reoccur after methadone dose reduction. This dysphoria could have been caused by high initial doses of methadone or accumulation due to frequent redosing. Therefore, based on individual patient evaluation reducing in methadone or extending application intervals could likely assist in prevention of dysphoria. Although not evaluated here, a reduced treatment frequency also might reduce stress and discomfort of patients due to reduced handling and might increase compliance with treatment in a busy clinical team. Furthermore, subcutaneous drug administration avoids the necessity of keeping a patent intravenous catheter and therefore can prevent possible painful incidents such as thrombophlebitis. This could also be avoided via the use of a transdermal fentanyl solution, which did prove to be effective and safe for the patients studied in the current trial. However, this specific fentanyl solution has been withdrawn from the market between the execution of the clinical trial and manuscript preparation and is currently (2025) not available. Fentanyl patches designed for application on human skin are still available, but owing to their different pharmacological properties, the results of this study cannot be transferred to the use of human fentanyl patches on dogs.
Methadone was administered four times daily over the course of the study, independent of the pain score of the animal. This alteration to clinical practice was taken to enable comparison to the long-acting transdermal fentanyl solution throughout the entire study. Therefore, the individual lowest effective dose was not titrated, and it might be, that some dogs in M received more methadone than they would have needed based on pain scoring. In a low number of dogs in group M mild unwanted gastrointestinal effects were present. It might be that they were caused by (to) high plasma levels of methadone. But the reason for these gastrointestinal effects cannot be determined with security. As vomiting and diarrhoea also occurred in single dogs in group F other factors like stress due to hospitalisation or changes in food compared to at home, could have caused them. The four-time daily subcutaneous methadone protocol seems to be suitable and safe for the majority of dogs after thoracolumbar spinal surgery. A dose reduction or extension of application intervals might be needed in individual dogs.
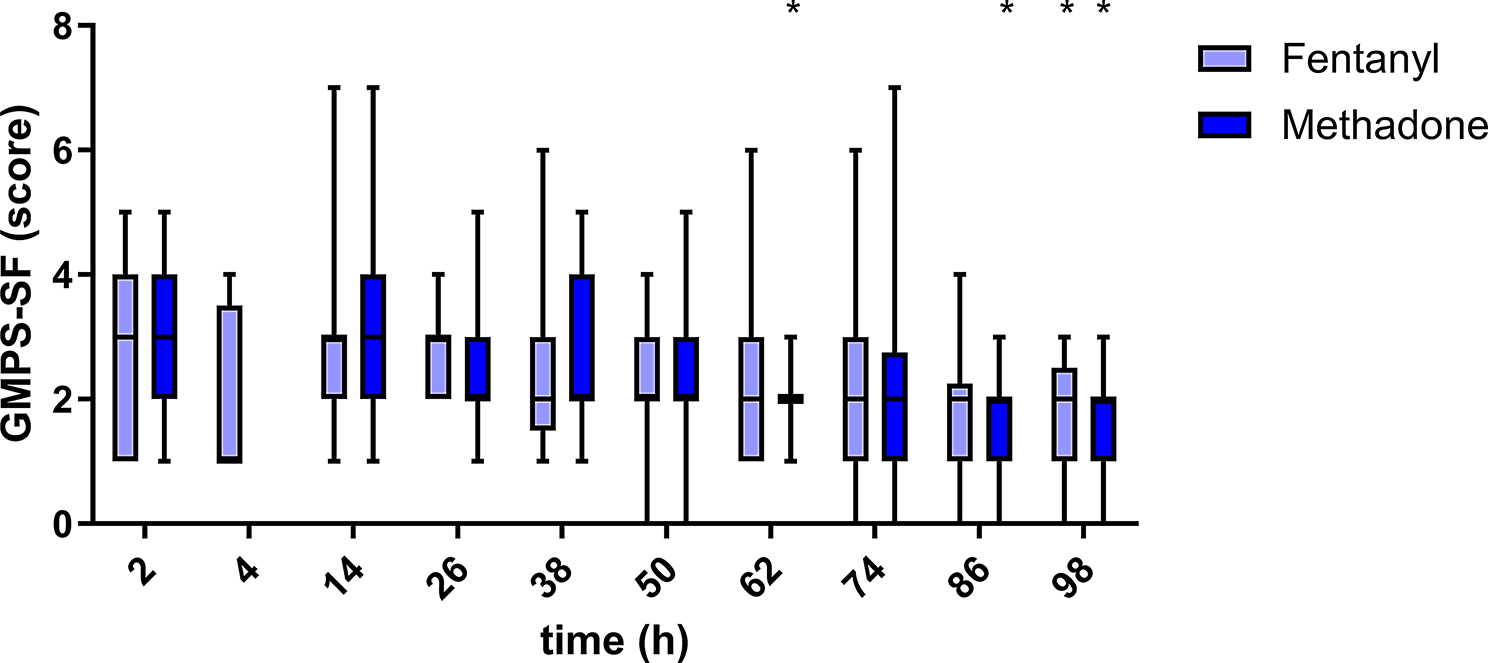
At present, no specific pain scale for evaluating dogs after neurosurgery is available; therefore, three different scales for the measurement of acute postoperative pain have been applied and compared. All three scales were able to detect postoperative pain behaviour, but the correlation between the two composite pain scales (CMPS-SF and CPS) was greater than that with the VAS. The VAS is considered a unidimensional scale that is based on the subjective impression of pain of the investigator [14, 15]. Hence, a very individual impression of the multimodal nature of pain is given. Both composite scales include three categories of evaluation: (a) observation of demeanour and posture, (b) approach to the animal and interaction, and C) touching the animal/painful area [36]. Following the steps of the scales, the observer is guided in how to evaluate possible pain; therefore, more aspects of pain behaviour are incorporated into the judgement in a structured way. This could explain why the composite scales correlate with each other more than with the VAS. Despite some differences in wording and the exact aspects that are evaluated, the CMPS-SF and CPS agreed concerning the outcome-based measure “Is rescue analgesia or more analgesia needed?” in 95% of all measurements. Considering the different intentions for which the scales were built, this might be of particular interest for institutions regularly working with untrained staff ([nurse] students, young veterinarians and nurses). The CMPS-FS was designed as a clinical decision-making tool, helping, together with the clinical judgement, to decide if an alteration in analgesic treatment is necessary [16]. In contrast, the CPS, which includes more visual aids, was created as a teaching tool, helping veterinary students identify behaviours, which could be caused by pain [37]. Keeping in mind that, in the present study, dogs were evaluated by a single person with moderate experience, both composite scales seem to reliably detect pain after neurosurgery and likely could be used interchangeably in these cases. The use of a scale that best fits the clinical situation (e.g., teaching hospital or not) therefore seems reasonable.
Von Frey Filaments were used to include a way of assessing dermal sensitivity and the presence of possible hypo- or hyperesthesia in the present study. The filaments at which the dogs responded were highly variable. Approximately 40% of the dogs responded only at one of the thickest filaments or not at all.
No distinct reason for this late reaction can be given. Dissection of nerves in the field of surgery could be one explanation, but using skin or skin and muscle incisions together with von Frey Filament application is an established model to evaluate postoperative analgesia, and a decrease in the reaction threshold is usually expected [38,39,40]. In addition, dermatomes often overlap; therefore, cutting the skin branch of a single spinal nerve should not result in increased thresholds [41]. Further, impairment of sensory neuron function on the level of the spinal cord, coursed by the disc disease could possibly also lead to reduced sensitivity. Despite the absence of statistical significance, a trend towards increasing sensitivity towards the end of the observation period was visible in the current study. The reason for this is not clear, but it might indicate the return of skin sensitivity. Furthermore, classical von Frey Filaments are used to detect changes such as hyperalgesia, which might develop after an insult [42]. Evaluating the dogs the first days after surgery might be too short for hyperalgesia or allodynia to be present. Further, the drugs applied could have influenced the results of von Frey Filament testing. Using an electronic von Frey device, an increase in thresholds after morphine application in dogs has been documented [43]. In addition to the µ-opioid effect, methadone acts antagonistically on spinal NMDA receptors, which could lead to an antihyperalgesic effect [7]. Pregabalin was applied to all animals in the present study. For pregabalin reducing cold and mechanical hyperalgesia in dogs with syringomyelia, chiari-like malformation or intervertebral disc disease has been demonstrated [17, 18]. Von Frey Filament testing was always the last step in evaluating a dog. Therefore, it cannot be excluded that some type of learning effect developed, leading to lower or later reactions of the dogs.
Nevertheless, the findings of the present study were similar to those reported in healthy dogs of ≤ 8 kg [44], which presented a response rate to von Frey Filament application in the thoracolumbar region of 56% and a tendency toward reactions with thicker filaments. Together with the fact that no clear hints for the need for rescue analgesia could be drawn from the data of the von Frey Filament tests, it is questionable whether the use of a test for skin hyperesthesia is a good choice for evaluating immediate postoperative analgesia. Perhaps measurements of classical mechanical thresholds via algometry would have been more useful.
The clinical nature of this trial causes some limitations of the study. The duration of disease before consultation in the clinic was not standardised; therefore, dogs with acute, subacute and chronic problems were included. This might have resulted in a heterogeneous study population but reflects the clinical situation. Pre-surgical pains scores as a baseline measure were not collected. They could be taken as an indicator of the severity of pain of the ongoing disease. But due to the clinical nature of the trial another impairment is that the dogs were enrolled independently of the analgesic treatment applied by the referring colleague. Therefore, it would have been questionable if a baseline pain score would really picture the baseline severity of pain or rather the efficiency of the already applied analgesic treatment. It remains unclear if and to what extent this medication could have influenced the results of postoperative patient evaluation. However, the drugs applied by the referring veterinarians most often were nonsteroidal anti-inflammatory drugs or steroids, which are likely not effective for longer than 24 h after application. Therefore, only the first measurement times might be influenced. Furthermore, including all possible cases reflects everyday practice and shows that the studied analgesic protocols appear to be effective and safe independent of previous medication. In addition, during the hospitalisation period, all medication, in addition to analgesic therapy, were at the discretion of the neurologist in charge and not standardised. All dogs included in this study received the parasympathomimetic bethanechol and the alpha sympatholytic phenoxybenzamine. For both drugs possible antinociceptive effects in laboratory rodents were discussed decades ago. However, whether these two drugs influenced pain modulation during the current trial cannot be proven. As no differences between the treatment groups and the applicability of the pain scores could be demonstrated, it appears highly unlikely that the additional medications have influenced the study results meaningfully.
All the animals were evaluated by only one investigator; to reduce stress to the hospitalised dogs, it was decided to refrain from examination by multiple assessors.