Study design. We conducted a retrospective study that included a collection of quantitative data from the health facility Tb treatment register using a data abstraction tool, and we also collected additional information relating to the patients from the Client Care cards. The data was accessed and collected by 15th March 2025. All Individuals with Pulmonary Bacteriologically Confirmed (PBC) and Pulmonary Clinically Diagnosed (PCD) Drug Susceptible Tb coinfected with HIV who started TB treatment from January 2020 to December 2021 were included in the study. Data on Individuals with treatment outcomes were considered. The findings were reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [11].
Study setting
The study was conducted in all Twenty-two (22) accredited diagnostic and Tuberculosis Unit health facilities located in 11 districts of the Teso region that include Katakwi, Amuria, and Kapelebyong, bordering Karamoja and have pastoralist communities. Kaberamaido, Serere, Kalaki, and Kumi are surrounded by water bodies, leading to significant engagement in fishing activities. Moreover, Bukedea, Kumi, Ngora, Soroti City and District, Kaberamaido, and Kalaki are all intersected by the Great North Road, resulting in frequent population movement and non-resident populations seeking healthcare within the region.
The combined population of these districts was estimated to be 2,462,344 in 2024 [12], with one of the lowest HIV/AIDS prevalence rates in Uganda at 4.2% [13]. Additionally, this is the fifth poorest region in Uganda, with a multidimensional poverty rate of 56%, 10.7% of the population have ever had an acute respiratory infection, and 5.2% have moderate smoking behavior [14,15,16]. The districts are largely rural and the majority of residents are subsistence mixed farmers, keeping goats and cattle and tending crops for a livelihood.
The health facilities were purposively selected because they were accredited to offer TB treatment services in the Teso region. Each facility serves as the TB diagnostic and treatment unit (DTU). Within each TB unit, there is a TB focal person who is either a nurse or a clinical officer. The facility focal persons are supervised by the District TB and Leprosy Supervisor (DTLS), an expert responsible for TB treatment and management at the district level. All these Health facilities receive support from the AIDS Information Center (AIC), funded by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) to enhance high-quality TB/HIV services through a health system strengthening grant to implement.
Inclusion and exclusion criteria
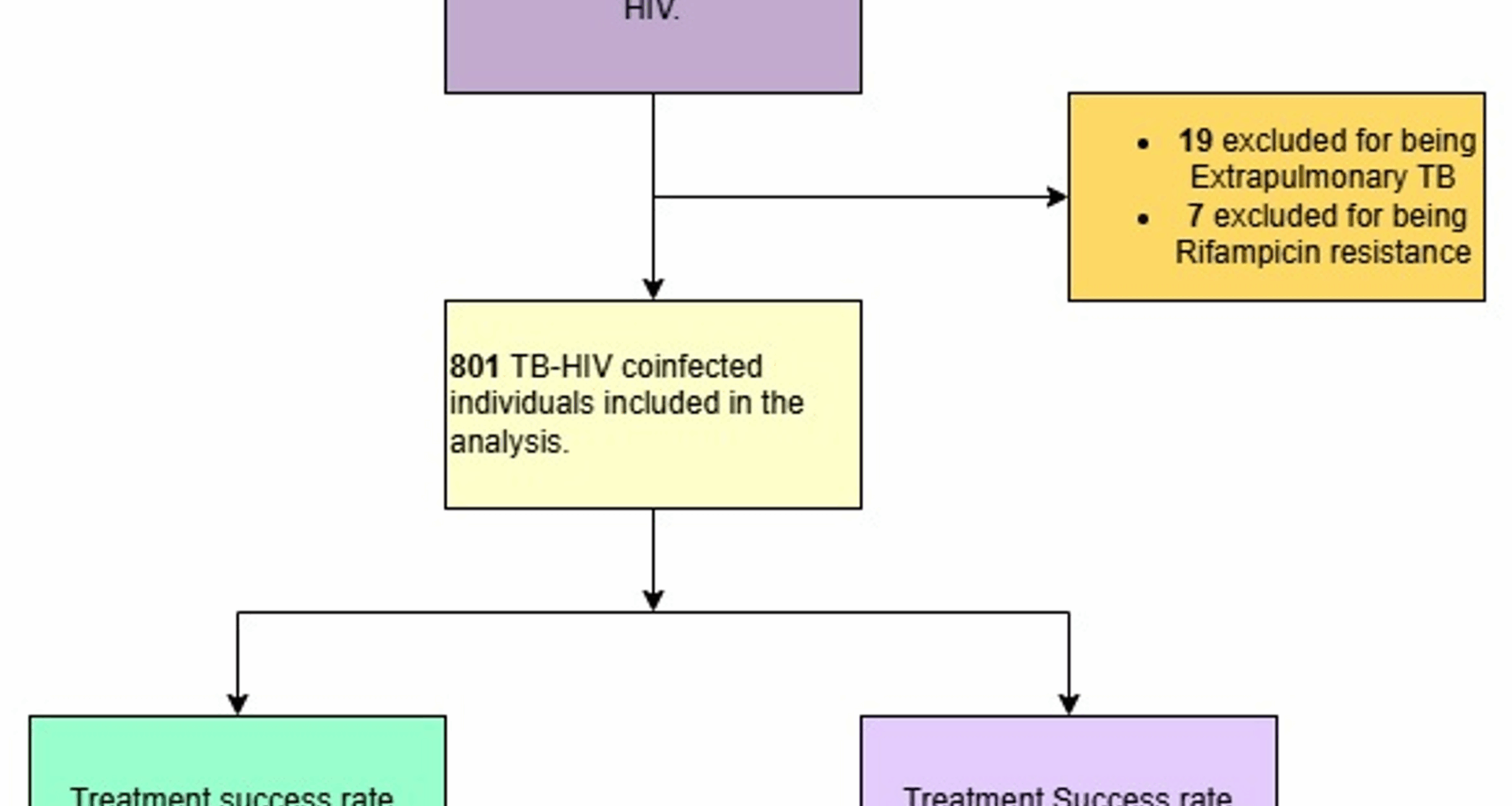
All individuals notified with Pulmonary Bacteriologically Confirmed and Clinically diagnosed Drug Susceptible Tb patients with a documented HIV Positive status in the Unit TB registers were included in the study. Individuals reported in the 22 Targeted DTUs from January 2020 to December 2021 were included in the study.
Individuals with Extra-pulmonary TB of any form, those with Drug-resistant TB, and those who were reported to be HIV-negative or of unknown HIV status were excluded from this study.
Study variables
Exposures: Demographic characteristics (age, gender), disease classification categorized as bacteriologically confirmed (laboratory diagnosis with urine LAM, Gen Xpert or Microscopy), clinically diagnosed (based on clinical assessment and chest x-ray diagnosis), and [17]TB treatment type categorized as new or retreatment, point of referral (Facility or community), GeneXpert results (Positive, Negative, and unknown).
The primary outcome was TB treatment Success is defined as treatment completion (completing treatment without evidence of failure but with no record to show that sputum smear or culture results in the last month of treatment and on at least one previous occasion were negative, either because tests were not done or because results are unavailable) and/or Cure (negative smear microscopy at the end of treatment and on at least one previous follow-up test at 2 months or 5 months) [18].
Treatment failure is when an individual remained smear-positive after 5 months of treatment, defaulted (patients who interrupted their treatment for two consecutive months or more), died, Lost to follow-up (A Tb patient who did not start treatment or who completed more than 1 month of treatment and was interrupted for 2 consecutive months or more) [18].
The HIV positive was determined as individuals with drug-susceptible TB having an HIV-positive test result (both the newly tested positive and the Known HIV-positive; individual already confirmed to be living with HIV as documented in the TB unit register) at the time of TB treatment initiation after TB diagnosis by either GeneXpert, urine Tuberculosis lipoarabinomannan or clinically diagnosed by a health worker [19, 20].
Data management and analysis
Using a designed data abstraction tool, data were extracted on both the exposure and outcome variables from the health unit TB registers from the time started on TB treatment until the treatment outcome was reached. The collected data was entered into a Microsoft Excel sheet and then exported to Stata Statistical Software: Release 15 (College Station, TX: StataCorpLLC.) where it was cleaned, validated, and analyzed [21].
Descriptive statistics of the study participants were summarized using proportions for categorical variables, means, and standard deviations for continuous variables with normal distribution and medians with interquartile ranges (IQRs) for continuous variables with skewed distributions. The association between treatment success (Yes/No) and the categorical variables were determined using the Chi-square or Fisher exact test, and associations between treatment success and numerical variables were assessed using the student t-test or Mann–Whitney U. Binary logistic regression analysis was conducted to determine factors associated with treatment success as adjusted odds ratios [22]. All variables significant (p p