Adenomyosis is a prevalent benign gynecological condition, with its incidence on the rise, particularly among younger women. The primary manifestations include dysmenorrhea and excessive menstrual blood volume, both of which can markedly impair daily activities. Early diagnosis is essential to avoid or delay surgery, enable medical management of the most severe symptoms, and preserve future fertility. The primary therapeutic objectives for adenomyosis management focus on alleviating dysmenorrhea, reducing menstrual blood volume, and enhancing the quality of life [4]. For young women, goals of therapy also includes preserving future fertility [5]. LNG-IUS containing 52 mg of levonorgestrel delivers levonorgestrel continuously into the uterine cavity, inhibiting the production of prostaglandins and thromboxanes, while simultaneously enhancing Fas antigen expression and reducing Bcl-2 protein levels, thereby mitigating dysmenorrhea and menstrual blood volume [6]. LNG-IUS containing 52 mg of levonorgestrel had been FDA-approved for the treatment of adenomyosis. LNG-IUS slowly releases levonorgestrel at a rate of 20 µg/day, exerting local progestin effects. It has no significant impact on blood lipids, blood sugar, or bone density, preserves female fertility, and allows for the restoration of reproductive capacity upon removal. Evidence suggests that LNG-IUS is more effective than danazol and GnRH-α in managing adenomyosis symptoms [7]. Additionally, studies have indicated that LNG-IUS provides similar symptomatic relief to hysterectomy, with superior improvements in quality of life scores [8]. LNG-IUS has thus emerged as a long-term, safe, and effective conservative treatment for adenomyosis [3]. In this study, patients reported significant relief from dysmenorrhea and a reduction in menstrual blood volume following LNG-IUS placement, aligning with findings from prior studies.
Patients with adenomyosis commonly present with enlarged uterine volume and increased menstrual blood volume. Due to Mirena’s singular size, relatively soft wings, and poor fixation, the likelihood of displacement and expulsion is greater in adenomyosis patients compared to those using contraceptives under normal conditions. Park [9] reported an expulsion rate of 37.5% in adenomyosis patients using LNG-IUS. In the present study, the expulsion rate in the conventional placement group was 33.3%. This outcome may be attributed to the enlarged uterine cavity, abnormal myometrial contractions, increased menstrual blood volume, a rigid endometrium, and cervical laxity in patients with adenomyosis, which aligns with findings from earlier studies [9]. While GnRH-α pretreatment has been shown to reduce expulsion rates by 40% [10], the associated costs warrant further evaluation. Long-term use of GnRH-α, however, can result in side effects such as estrogen deficiency, perimenopausal syndrome, and osteoporosis [11], and the expulsion rate may recur upon discontinuation of GnRH-α. Additionally, one study indicated that despite GnRH-α pretreatment, the expulsion rate remained high at 21.8%, with approximately one-third of patients opting to replace their LNG-IUS and over half selecting alternative treatment methods [10]. The expulsion of LNG-IUS not only undermines treatment efficacy but also increases financial burdens and patient anxiety. In response to displacement and expulsion, Zhu [12] first introduced hysteroscopic LNG-IUS suture fixation in a patient with a history of LNG-IUS expulsion. Subsequently, Cui [13] and others reported successful instances of hysteroscopic suture fixation of LNG-IUS. In the present study, the expulsion rate in the hysteroscopic suture fixation group was 4.8%, which was markedly lower than in the conventional placement group, further confirming the efficacy of hysteroscopic suture fixation in reducing displacement and expulsion rates. An analysis of the single case of LNG-IUS expulsion after hysteroscopic suture fixation revealed that the patient was in the mid-menstrual cycle, with an endometrial thickness of 9 mm and insufficient suture depth during the procedure, Likely contributing to the expulsion. Therefore, hysteroscopic Mirena suture fixation requires skill and experience. The optimal timing for suture placement is three days post-menstruation when endometrial tissue is minimal, and visibility during surgery is clearer. Preoperative assessment of uterine cavity morphology and structure is vital. A 1/2 arc circular needle with a chord length of 20 mm or less should be used, with moderate force applied when clamping sutures to prevent breakage. Sutures should be placed near the fundus into the superficial muscle layer, as excessive depth complicates needle withdrawal, while insufficient depth raises the risk of post-operative expulsion. Gentle knotting is essential to prevent suture breakage and myometrial tissue damage. Finally, maintaining appropriate distension pressure is crucial for ensuring proper uterine wall flattening. Although hysteroscopic Mirena suture fixation offers conservative treatment options for adenomyosis patients with prior LNG-IUS expulsion, side effects, such as irregular vaginal spotting, may arise. This spotting is possibly linked to the exposure of small vessels and an increase in αSMA-covered vessels following LNG-IUS placement [14]. In the current study, the post-operative spotting rate in the hysteroscopic fixation group was 38.1%, which is similar to the findings of Nengyuan [15], who reported abnormal uterine bleeding (44.3%) after hysteroscopic placement and comparable to the conventional placement group. Sheng [16] noted that the frequency of spotting gradually decreased over time after Mirena’s placement. Due to the limited follow-up period in this study, further research is planned to observe the resolution of spotting over a longer duration.
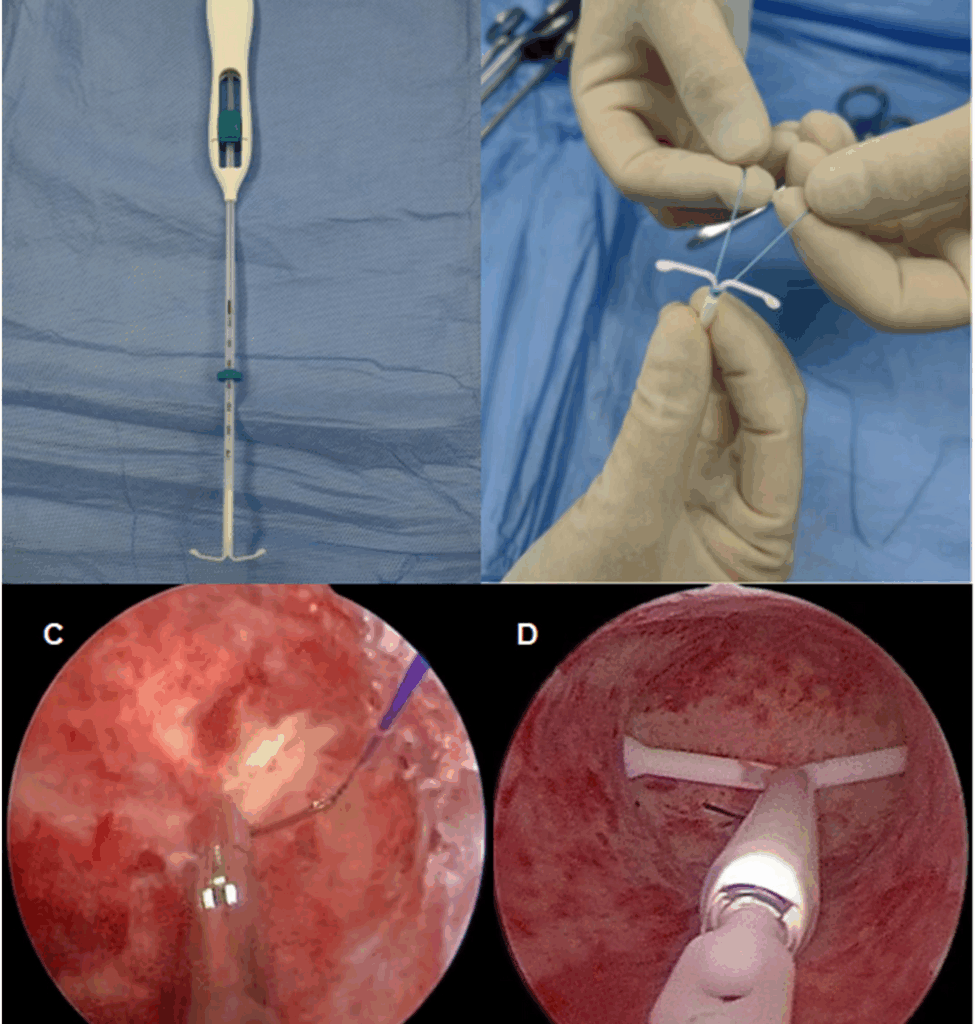
Hysteroscopic cold knife suturing is a minimally invasive technique performed via natural body cavities. Under the high-definition view provided by hysteroscopy, intrauterine conditions are clearly visible, enabling precise suturing under direct observation. The procedure does not necessitate specialized instruments, which simplifies the learning process for clinicians. Surgeons with experience in laparoscopic suturing are capable of performing this procedure, which typically requires less than thirty minutes for completion. Huang [17] et al. demonstrated that surgeons with expertise in single-port laparoscopic suturing were able to notably shorten the time required for hysteroscopic suturing. Furthermore, no complications, such as water intoxication, electrolyte imbalance, or uterine perforation, were encountered during the hysteroscopic suture fixation procedure. Furthermore, the procedure did not lead to an increase in postoperative abdominal discomfort, which suggests a favorable safety profile. These outcomes align with the findings reported by Mao [18] et al.
While no complications were observed in this study, prolonged procedure time could potentially increase risks of thrombosis, infection, and anesthesia-related adverse events. Notably, device removal requires hysteroscopic visualization to identify and release the fixation sutures under anesthesia prior to standard IUS extraction. This process extends removal time by several minutes compared to conventional LNG-IUS removal and substantially increases procedural costs.
In conclusion, hysteroscopic LNG-IUS suture fixation effectively mitigates device expulsion by anchoring the LNG-IUS to the uterine wall. The fixation effect is more precise, and the surgical treatment outcome is remarkably effective.This fixation not only alleviates dysmenorrhea but also decreases menstrual blood volume in patients with adenomyosis, offering a novel conservative treatment option for those with a history of LNG-IUS expulsion. The technique holds considerable potential for broader clinical application. However, given the limited sample size in this study, further research with larger cohorts is required to assess the efficacy and feasibility of hysteroscopic LNG-IUS suture fixation in the treatment of adenomyosis.