Lactobacillus delbrueckii inhibits the growth of MRSA
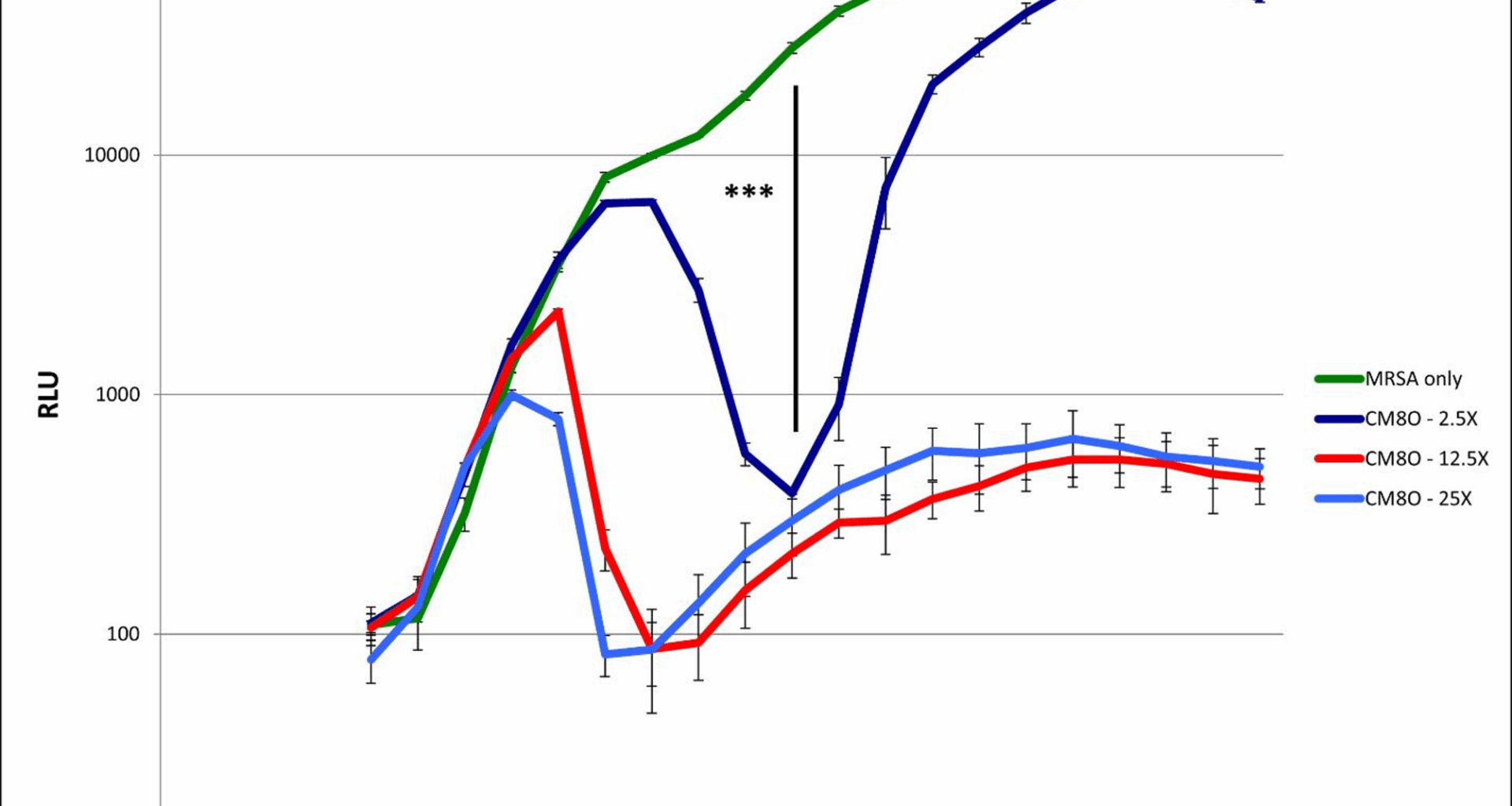
We first examined the effect of L. delbrueckii on the growth of Xen31 cells in our co-culture assay. The presence of CM80 resulted in decreased MRSA growth by the detection of bioluminescence (Fig. 1). CM80 on its own showed negligible growth under these co-culture conditions, and growth of an L. plantarum co-culture control had no effect on MRSA growth (data not shown). It appears that after approximately 3–5 h, some anti-Staphylococcal factor begins to decrease MRSA growth in a concentration-dependent manner. When approximately equal starting numbers of MRSA and CM80 are co-cultured, luminescence returns to control levels after approximately 10 h. However, at higher relative ratios of CM80 to MRSA, luminescence values remain depressed even after 13 h. We repeated this assay multiple times in triplicate and, at 13 h, we obtained colony-forming unit (cfu) counts to ensure that the decreased luciferase expression we observed was representative of an actual decrease in viable MRSA cell growth. This was done by serially diluting the co-culture samples in BHI media and plating on ChromAgar S. aureus plates. We found a > ten-fold reduction of MRSA in our co-culture model, confirming that the decreased luciferase activity we observed was in fact the result of greatly decreased bacterial growth (data not shown).
Co-culture growth of MRSA in the presence of the probiotic bacterium L. delbrueckii
A growth curve for MRSA was obtained in the co-culture assay by monitoring emitted light. MRSA alone (green) shows rapid growth to stationary phase. Inclusion of CM80 at varying concentrations (blue, red, and dark blue) shows inhibition of MRSA growth, which first appears at around 4 h of co-culture. At 6–6.5 h all co-cultures with CM80 show significant growth inhibition ( *** : p
Lactobacillus delbrueckii inhibits the growth of E. coli
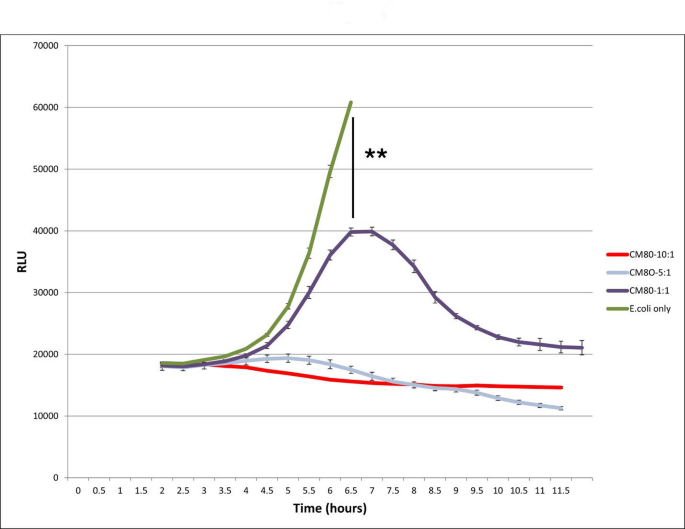
CM80 was then again used in our co-culture assays, but mixing fluorescent E. coli with increasing amounts of L. delbrueckii. As with MRSA, this resulted in decreased E. coli growth and accumulation, by the detection of total fluorescence (Fig. 2). Although GFP expression quickly saturates the assay at approximately 7 h when E. coli is grown by itself, the presence of 5-10-fold excess L. delbrueckii over E. coli results in a drop in fluorescence down to background levels. Again, this is a concentration- dependent phenomenon; at a 1:1 ratio of CM80/E. coli a lesser degree of inhibition was observed, but it is still present and strong. As with MRSA, we repeated this assay at 13 h and obtained cfu counts to ensure that the GFP expression was representative of E. coli growth. Colony plating of co-cultures with 5- and 10-fold excess CM80 showed essentially complete negation of E. coli growth in our co-culture model, with no detectable colonies at any dilution when samples were serially diluted and plated on LB agar plates plus chloramphenicol.
Co-culture growth of E. coli in the presence of L. delbeuckii
Fluorescent E. coli was incubated with increasing amounts of the probiotic bacterium, L delbrueckii (CM80); growth curves for E. coli were obtained by quantitating the fluorescence of the culture. E. coli alone (green) shows rapid logarithmic growth. Inclusion of CM80 (purple, grey, and red) shows inhibition of E. coli growth. At a lower loading ratio (1:1), this inhibition first appears around 6 h of co-culture and is significant ( ** : p
Pathogen inhibition by Lactobacillus delbrueckii is mediated by an induced peptide
The apparent delay in the onset of growth inhibition by CM80 led us to suspect that the bacterium was acting through an induced mediator, likely a bacteriocin peptide as has been described for other probiotic microbes [10]. We therefore next sought to determine if the induced anti-pathogenic agent was a peptide. We set up a co-culture of L. delbrueckii and MRSA in 100 mL LB media, collecting fractions every hour for 12 h; fractions were prepared by removing 1 mL of culture, centrifugation at 16,000*g for one minute, and the supernatant collected. Equal volumes of 50 µL supernatant (conditioned media) and 5*104 cfu MRSA in 50 µL 2X LB were then mixed and incubated at 37 °C, and assayed for a decrease in absorbance in a 96-well format. In this assay, reduced absorbance would be an indicator of inhibited bacterial growth.
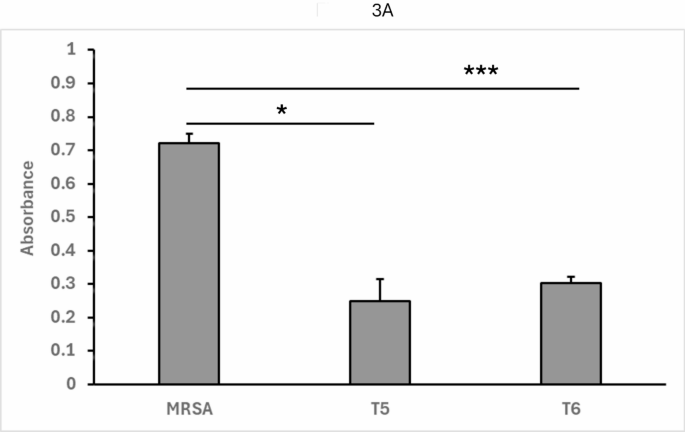
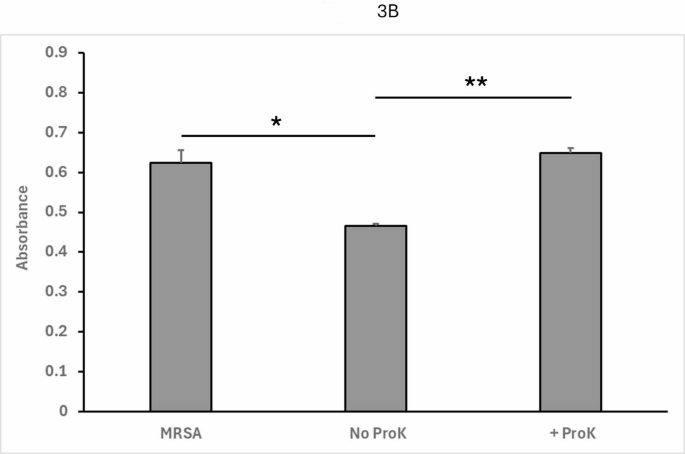
As seen in Fig. 3A, fractions collected after 5–6 h of co-culture resulted in a large decrease of absorbance compared to MRSA-only control, indicating successful inhibition of pathogen growth by the conditioned media alone. The strongest fraction, fraction 6, was then pre-treated with Proteinase K (ProK) for 1 h at 50 °C (or left untreated as a control). Pre-treated and untreated control samples of fraction 6 were then again tested in the assay as described above. As seen in Fig. 3B, the proteinase K-treated fraction resulted in no impaired growth of MRSA, indicating that the induced agent is sensitive to proteolytic degradation, consistent with a bacteriocin peptide.
A co-culture inducible factor responsible for pathogen inhibition is sensitive to proteolysis
3A: MRSA and L. delbrueckii were co-cultured at scale, collecting fractional aliquots every hour. The conditioned media of these fractions was then mixed with MRSA and incubated at 37 °C. Cultures were assayed for a decrease in absorbance at 600 nm in a 96-well format. T5 = 5 h. sample. T6 = 6 h. sample. Data shown represent the mean and standard deviation from at least four independent determinations. MRSA = MRSA alone (no conditioned medium). ( * : p *** : p
The results above show that MRSA alone shows robust growth in the TECAN assay. However, when fractions of conditioned medium from 5 h to 6 h of co-culture with CM80 are added, growth is markedly inhibited. This shows that the presence of living probiotic bacteria is not required for an inhibitory effect on pathogen growth.
3B: Fraction 6 was either treated with ProK or mock-treated (No ProK) and then incubated with MRSA in the same 96 well co-culture assay.
MRSA alone again shows robust growth in the TECAN assay (left). When fraction 6 control (No ProK) is present, there is again marked inhibition of growth (middle). When fraction 6 is pre-treated with proteinase K (ProK), this inhibition disappears (right). These results indicate that the inhibitory molecule in the conditioned medium is proteinaceous, consistent with a bacteriocin peptide. Absorbance was measured at 600 nm. Data shown represent the mean and standard deviation from at least four independent determinations. ( * : p ** : p