Identification of DlGRAS family genes
In this study, 56 GRAS genes were identified in longan and named DlGRAS1–DlGRAS56 according to their physical location on the chromosome (Fig. 3A). The fundamental properties of the 56 DlGRAS proteins (Supplementary Table 1), including the number of amino acids, molecular weight (MW), isoelectric point (pI), instability index, and subcellular localization, were examined in this study. The number of amino acids encoded by GRAS gene of longan was between 87 and 822. Their molecular weights were 9.70 to 93.32 kDa. The range of the isoelectric points was 4.56 to 9.42. DlGRAS15, DlGRAS23, DlGRAS26, DlGRAS28, DlGRAS29, DlGRAS31, and DlGRASA43 were the only stable proteins in this family (instability coefficient: DlGRAS family were primarily found in the cytoplasm, mitochondria, nucleus, and plasma membrane.
Phylogenetic analysis of DlGRAS family members
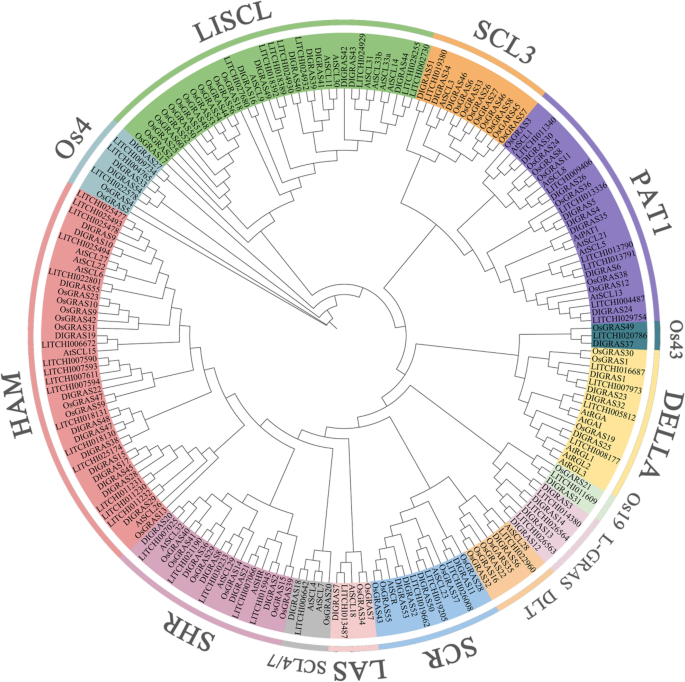
A phylogenetic tree was constructed using 33 previously identified Arabidopsis thaliana GRAS (AtGRAS) genes, 60 Oryza sativa GRAS (OsGRAS) genes, 55 litchi (Litchi chinensis Sonn.) GRAS (LcGRAS) genes, and 56 longan (Dimocarpus longan Lour.) GRAS (DlGRAS) genes (Supplementary Table 2).The GRAS genes in longan were classified into 14 subfamilies, with the distribution of members as follows: the SCR subfamily contained 4 members; the SCL3 subfamily included 3 members; the DLT, SCL4/7, LAS, Os19, and Os43 subfamilies each have 1 member; the DELLA subfamily comprised 4 members; the LISCL subfamily had 8 members; the HAM subfamily consisted of 13 members; the Os4 subfamily contained 3 members; the SHR subfamily included 5 members; and the PAT1 subfamily had 7 members. Additionally, four longan genes, namely DlGRAS3, DlGRAS12, DlGRAS13, and DlGRAS14, are assigned to the L-GRAS subfamily (Fig. 1). The L-GRAS subfamily contained 4 members of the longan DlGRAS family, suggesting that this litchi-specific subfamily may have unique functions in longan, litchi, or their close relative species.
Phylogenetic tree of GRAS family members in longan (Dl), Arabidopsis (At), rice (Os) and litchi (Lc). The Evolutionary tree was constructed using MEGA 7.0 and using the Maximum likelihood method with the 1,000-times bootstrap test. The fourteen groups are represented with different colors
Gene structures and composition of motifs and domains
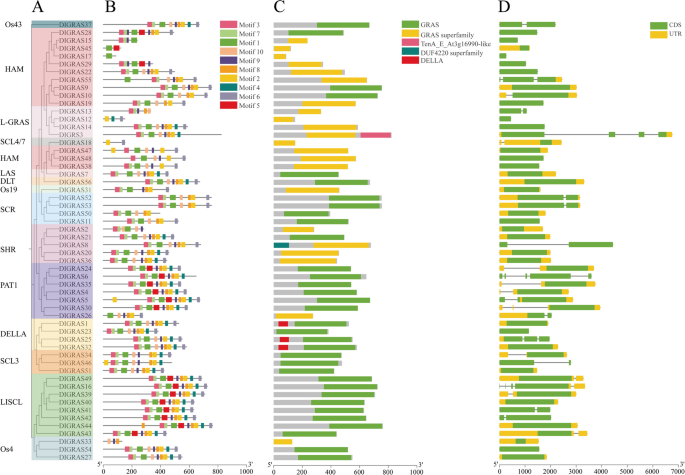
Longan GRAS proteins were found to include ten comparatively conserved motifs (Fig. 2A, B), with a larger number of these motifs found at the C-end than at the N-end. We have identified 10 motifs, corresponding to LHR I (motifs 4 and 8), VHIID (motifs 1 and 5), LHR II (motifs 10 and 9), PFYRE (motifs 7 and 2), and SAW (motifs 3 and 6) (Supplementary Tables 3 and Supplementary Table 4). Motif1, Motif2, Motif4 and Motif8 existed in most DlGRAS, and only one conserved motif was present in DlGRAS17 (Motif1). Although the distribution of the conserved motifs within each subfamily was similar, the composition of conserved motifs in DlGRAS differed significantly among the subfamilies. Nonetheless, DlGRAS genes from different subfamilies have distinct conserved motifs. For example, DlGRAS8 in the SHR subfamily contained motif 6 and DlGRAS6 in the PAT1 subfamily contained mostly Motif4. Motif7 was present in the majority of DlGRAS44 genes in the LISCL subfamily. Additionally, this study also found that motif 5 was only present in the PAT1, HAM, and LISCL subfamilies.
All DlGRAS members contained GRAS domains and members of the same subfamily contained the same conserved domains (Fig. 2C). For example, three of the four members of the DELLA family contained DELLA-conserved domains, whereas members of the PAT1 subfamily had GRAS conserved domains. The most GRAS conserved domains were located at the C-end. DlGRAS8 had a DUF4220 superfamily conserved domain, and DlGRAS3 had a TenA E At3g16990-like conserved domain.
In the structure of the DlGRAS gene, twenty-five genes contained introns. All PAT1 family members contain introns in their DlGRAS structures. DlGRAS6 and DlGRAS16 contain four introns, DlGRAS3 and DlGRAS55 contain three introns, and DlGRAS25 and DlGRAS30 contain two introns (Fig. 2D). DlGRAS4, DlGRAS5, DlGRAS8, DlGRAS10, DlGRAS13, DlGRAS18, DlGRAS24, DlGRAS26, DlGRAS34, DlGRAS35, DlGRAS37, DlGRAS39, DlGRAS41, DlGRAS42, DlGRAS43, DlGRAS46, DlGRAS49, DlGRAS52 and DlGRAS53 each contained one intron, and the remaining DlGRAS genes contained no introns.
These results indicate that different subfamilies have distinct structural compositions and exhibit great functional diversity.
Gene structures and composition of motifs and domains in DlGRAS genes. A A tree of the GRAS members in Longan. B Distribution of motifs in DlGRAS proteins. C Domain of DlGRAS genes. D Exon–intron structures of the DlGRAS genes
Chromosomal distribution and collinearity analysis of DlGRAS
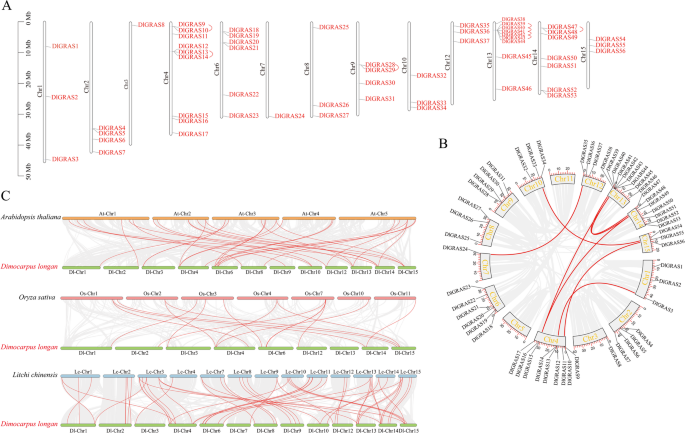
The distribution of GRAS genes on the longan chromosomes were also analyzed using the longan genome (Fig. 3A). They were densely distributed on chromosomes Chr4, Chr6, Chr13, and Chr14, with Chr4 and Chr13 each containing nine genes, representing the most abundant distribution. In contrast, only one gene was present on chromosomes Chr3 and Chr7, where the members were sparsely dispersed. To further explore the evolutionary relationships among GRAS family members, collinear analysis of the longan genome was conducted. Within the longan GRAS family, 13 genes located on Chr4, Chr9, Chr13, and Chr14 were found to have undergone tandem repeat events (Fig. 3A, Supplementary Table 5). Specifically, the genes on Chr4, Chr9, and Chr14 belong to the HAM subfamily, while those on Chr13 belong to the LISCL subfamily. Additionally, eight segmental duplication events were identified among 13 DlGRAS sequences (Fig. 3B, Supplementary Table 6). In summary, tandem duplication and segmental duplication may have played a major role in the evolution of the longan GRAS gene family.
A Schematic representations of the chromosomal distribution of DlGRAS genes. The red lines indicate duplicated GRAS gene pairs. B Chromosome localization of duplicated DlGRAS genes in longan. The red lines represent the segmentally duplicated genes, and the gray bands represent the collinear block. C Synteny analyses of the GRAS genes among longan, Arabidopsis, rice, and litchi. The syntenic GRAS gene pairs among longan, Arabidopsis, rice, and litchi are highlighted with red lines.The gray lines indicate the collinear blocks within the genomes of longan, Arabidopsis, rice, and litchi
Collinearity analysis was carried out for four plants (Longan, Arabidopsis, rice and litchi) in order to determine the evolutionary relationships of GRAS genes between various species (Fig. 3C, Supplementary Table 7). According to these findings, 31 gene pairs were found to be directly homologous in Arabidopsis and longan, 16 gene pairs were found to be directly homologous in rice and longan, and 54 gene pairs were found to be directly homologous in litchi and longan. Longan and litchi each have 18 non-homologous genes (Supplementary Table 8). Eighteen longan GRAS genes were located on five different longan chromosomes (Chr4, Chr9, Chr10, Chr13, Chr14), and eighteen litchi GRAS genes were located on ten different litchi chromosomes (Chr2, Chr3, Chr6, Chr8, Chr9, Chr10, Chr11, Chr13, Chr14, Chr15) (Supplementary Fig. 1). Furthermore, DlGRAS3, DlGRAS8, DlGRAS9, DlGRAS18, DlGRAS20, DlGRAS21, DlGRAS35, DlGRAS49, and DlGRAS55 have syntenic loci in Arabidopsis, rice, and litchi. These genes belong to the L-GRAS (DlGRAS3), SHR(DlGRAS8, DlGRAS21), HAM(DlGRAS9, DlGRAS55), SCL4/7(DlGRAS18), PAT1(DlGRAS35), and LISCL(DlGRAS49) subfamilies, respectively, suggesting that they may have conserved biological functions in plants.
Cis-acting element analysis of DlGRAS genes
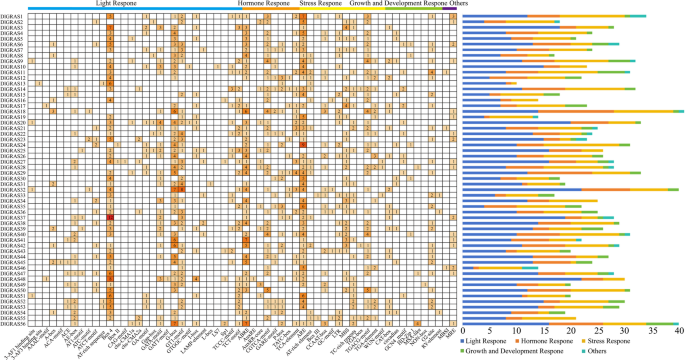
The upstream 2 000 bp promoter region of 56 DlGRAS gene were analyzed, and 1436 elements were obtained, including light, growth development, hormone, and stress response elements (Fig. 4). All members of the DlGRAS gene family contain photoresponse-related elements, 17 of which also contain MYB-binding sites that influence photoresponse-related (MRE) related elements. Auxin, salicylic acid, abscisic acid, methyl jasmonate, and gibberellin are five other cis-acting elements linked to plant hormones. The presence of these cis-acting elements suggests that these hormones regulate DlGRAS.
Distribution of cis-acting elements of DlGRAS family members. The different colors and numbers of grids indicate the numbers of cis-regulatory elements. The histograms on the right represent the sum of the cis-regulatory elements in each category.
Cis-acting elements linked to abiotic stressors, including anaerobic induction, drought stress, hypoxia-specific induction, low-temperature responses, trauma responses, and defensive stress responses have also been identified in the DlGRAS gene family. Cis-acting elements associated with seed specificity, endosperm expression, zein-olysin metabolism, palisade mesophyll cell differentiation, circadian rhythm control, MYB-binding site involvement in flavonoid biosynthesis, cell cycle regulation, and meristem-specific activation have also been identified in this family.
In summary, the most important function of DlGRAS family is the photoresponse, which strongly influences hormone responses and abiotic stress. Meanwhile, members of the longan GRAS gene family play important roles in photoperiod, circadian rhythm, and stress pathways, demonstrating the diversity of the functions of longan GRAS genes.
Expression analysis of DlGRAS genes in different tissues and organs
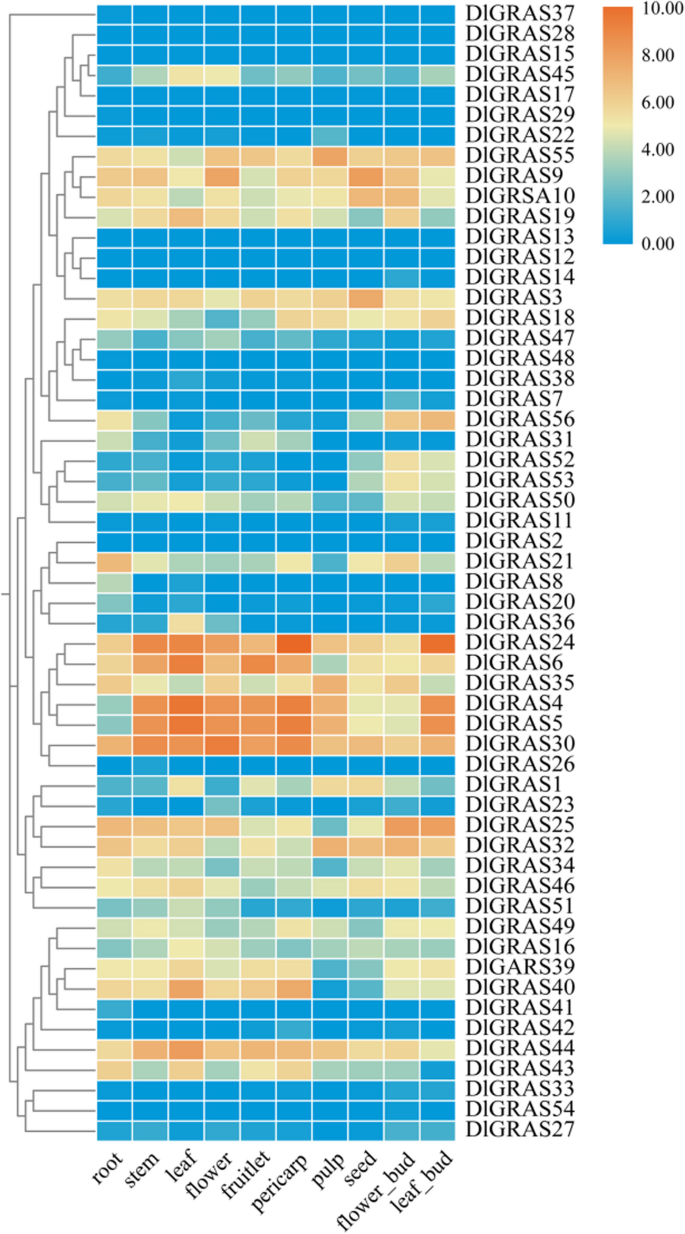
The transcriptional expression of the 56 DlGRAS genes was analyzed using data from the roots, stems, leaves, flowers, fruitlets, and seeds of longan (Fig. 5, Supplementary Table 9). DlGRAS genes were differentially expressed in various longan tissues. DlGRAS members from the same subfamily exhibited similar expression patterns. While most SHR family genes exhibited low expression levels across all tissues, most PAT1 family genes displayed high expression levels across all tissues. The stems and pulp showed higher levels of DlGRAS4, DlGRAS5, DlGRAS24, and DlGRAS30, whereas the leaves and fruitlets showed higher levels of DlGRAS4, DlGRAS5, DlGRAS6, and DlGRAS24. While DlGRAS24 was highly expressed in leaf buds, DlGRAS4, DlGRAS5, and DlGRAS30 were highly expressed in flowers.
Expression profiles of DlGRAS family genes in different tissues and organs of longan. The heatmap was generated using the FPKM values of DlGRAS from the transcriptome data (GSE84467). In the heatmap, orange indicates high expression, and blue indicates low expression (log2 (FPKM + 1))
Analyses of GRAS family gene expression in different longan varieties during flowering
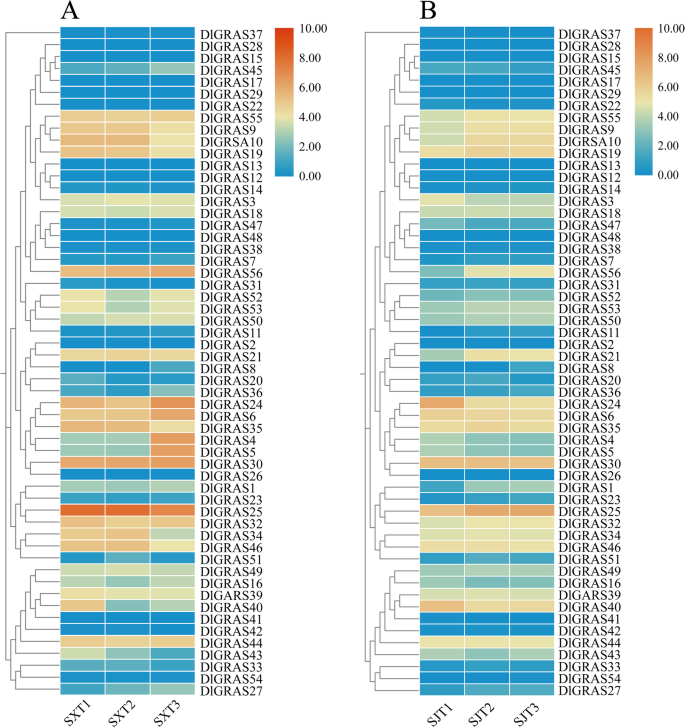
The expression pattern of DlGRAS genes at three flowering stages of ‘SX’ and ‘SJ’ were analyzed. The expression of the DlGRAS gene family in ‘SX’ longan was not significant during the T1-T2 stage, but some genes showed significant upregulation or downregulation during the T2-T3 stage (Fig. 6, Supplementary Table 10). The upregulated genes included DlGRAS4, DlGRAS5, DlGRAS6, DlGRAS24, DlGRAS36, and DlGRAS45, whereas the downregulated genes included DlGRAS10, DlGRAS34, DlGRAS35, DlGRAS43, DlGRAS46, and DlGRAS51. During the T1-T2 stage of ‘SJ’ longan, some genes showed significant upregulation or downregulation. The upregulated genes included DlGRAS1, DlGRAS10, DlGRAS11, DlGRAS14, DlGRAS21, DlGRAS25, DlGRAS27, DlGRAS51, and DlGRAS56, and the downregulated genes included DlGRAS22, DlGRAS24, DlGRAS40, and DlGRAS47. However, throughout the T2-T3 stage of ‘SJ’ longan, the expression levels of DlGRAS genes were not statistically significant. Additionally, among these 56 DlGRAS genes, DlGRAS24 was significantly downregulated by 4.3 times in ‘SJ’ during the T1-T2 stage, but no significant differential expression was observed during the T1-T2 stage in ‘SX’ longan. Based on the expression results obtained during longan flowering, we selected the DlGRAS24 gene for subsequent functional validation.
Expression patterns of DlGRAS genes in the three flowering periods of ‘SX’ and ‘SJ’. The heatmap was generated using the FPKM values of DlGRAS from the transcriptome data. In the heatmap, orange indicates high expression, and blue indicates low expression (log2 (FPKM + 1)). A Expression pattern of DlGRAS genes in three flowering periods of ‘SX’. B Expression pattern of DlGRAS genes in three flowering periods of ‘SJ’ longan. T1 represents the dormant stage (before the emergence of floral primordia), T2 represents the emergence of the floral primordium stage, and T3 represents the floral organ formation stage
Subcellular localization analysis of DlGRAS24
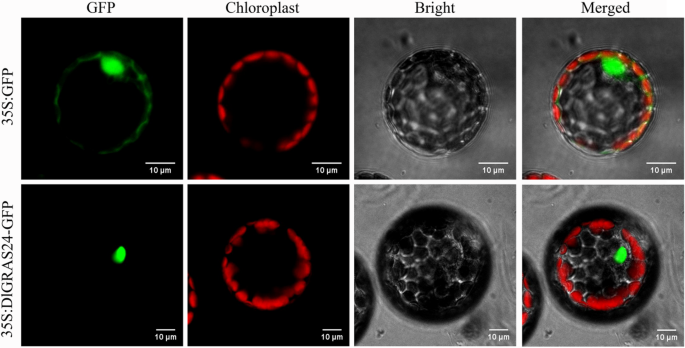
To detect the location of DlGRAS24 protein in cells, we constructed a fusion protein expression vector (35 S: DlGRAS24-GFP) containing enhanced green fluorescent protein (GFP), introduced it into Arabidopsis mesophyll protoplasmic cells using a PEG-mediated method, and observed it using laser confocal microscopy (Fig. 7). Under excitation at 480 nm, the fluorescence signal of 35 S: DlGRAS24-GFP was observed only in the nucleus, and no GFP signal was observed in the cytoplasm or cell membrane (Fig. 7). In contrast, the GFP signal was observed in whole cells of the 35 S: GFP control group, with no specific localization. This suggests that DlGRAS24 is localized to the nucleus.
Subcellular localization of DlGRAS24. 35 S: GFP was the empty vector. 35 S: DlGRAS24 – GFP shows the localization of the DlGRAS24 protein. GFP (green fluorescent protein distribution), Chloroplast (chloroplast localization), Bright (bright – field cell structures), Merged images of all three images. (Bar = 10 μm)
Phenotypic analysis of DlGRAS24 transgenic Arabidopsis
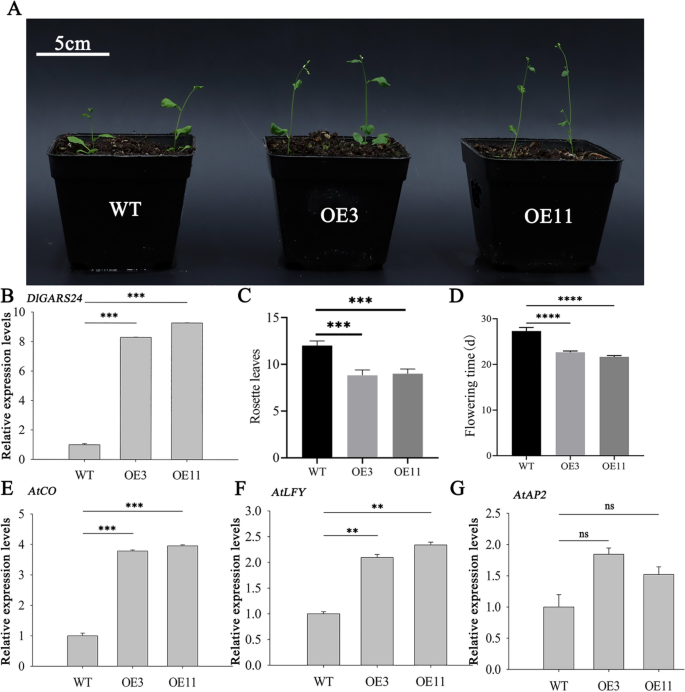
Two positive DlGRAS24-OE transgenic plant lines with high expression levels were chosen at random to examine phenotypic changes in the transgenic plants after DlGRAS24 was inserted into wild-type Arabidopsis for heterologous overexpression (Fig. 8A-B). Compared with wild-type plants, the flowering times of the transgenic strains were significantly different: the transgenic strains had bolted flowering at approximately thirteen leaves (approximately 23 d after germination), and the wild-type flowering time was 5 d later than that of the transgenic lines, showing more obvious early flowering (Fig. 8C-D, Supplementary Table 11).
To clarify the reasons for the early flowering of transgenic strains compared to that of the wild type, the expression levels of AtCO, AtLFY, and AtAP2 in the wild type and DlGRAS24-OE strains were analyzed using qRT-PCR. The expression levels of AtCO and AtLFY in transgenic Arabidopsis were significantly increased (Fig. 8E-F). AtAP2 showed no significant change (Fig. 8G). These results suggest that DlGRAS24 may promote flowering through its interaction with AtCO and AtLFY.
Heterotopic expression of the DlGRAS24 gene in Arabidopsis and expression analysis of AtCO, AtLFY, and AtAP2 in transgenic Arabidopsis. A Flowering of Arabidopsis with overexpression of the DlGRAS24 gene. B, E–G Relative expression of endogenous genes in Arabidopsis DlGRAS24 overexpression lines. C–D Number of rosette leaves and flowering time in Arabidopsis