Oxetanes and other four-sided molecular rings have become increasingly common in drug design thanks to their small size and ability to add three-dimensionality to molecules. There are lots of oxetane building blocks available from commercial vendors or in companies’ screening libraries for chemists to play with.
A new atom-swapping reaction developed by Ming Joo Koh and his team at the National University of Singapore could empower chemists to use oxetane fragments in new ways (Nature 2025, DOI: 10.1038/s41586-025-09723-3). The straightforward procedure can trade oxetane’s oxygen atom for a nitrogen, sulfur, or carbon atom—handy for streamlining synthetic routes, diversifying molecules, and whipping up new analogs of complex molecules with ease.
“You can really simplify synthesis in a big way” with this new reaction, Koh says. His overarching research goal is to come up with creative ways to make complex synthesis easier, which is one of the main reasons he joined the growing cadre of chemists working on atom swapping. While other groups focus on editing aromatic scaffolds, he and his team turned their attention to saturated rings.
The first step in the researchers’ new atom-swap process is to remove the oxygen atom. To do this, they adapted a light-driven method developed by Corey Stephenson’s lab (Nat. Chem. 2011, DOI: 10.1038/nchem.949) to crack open the ring and turn its two carbon-oxygen bonds into carbon-bromine bonds.
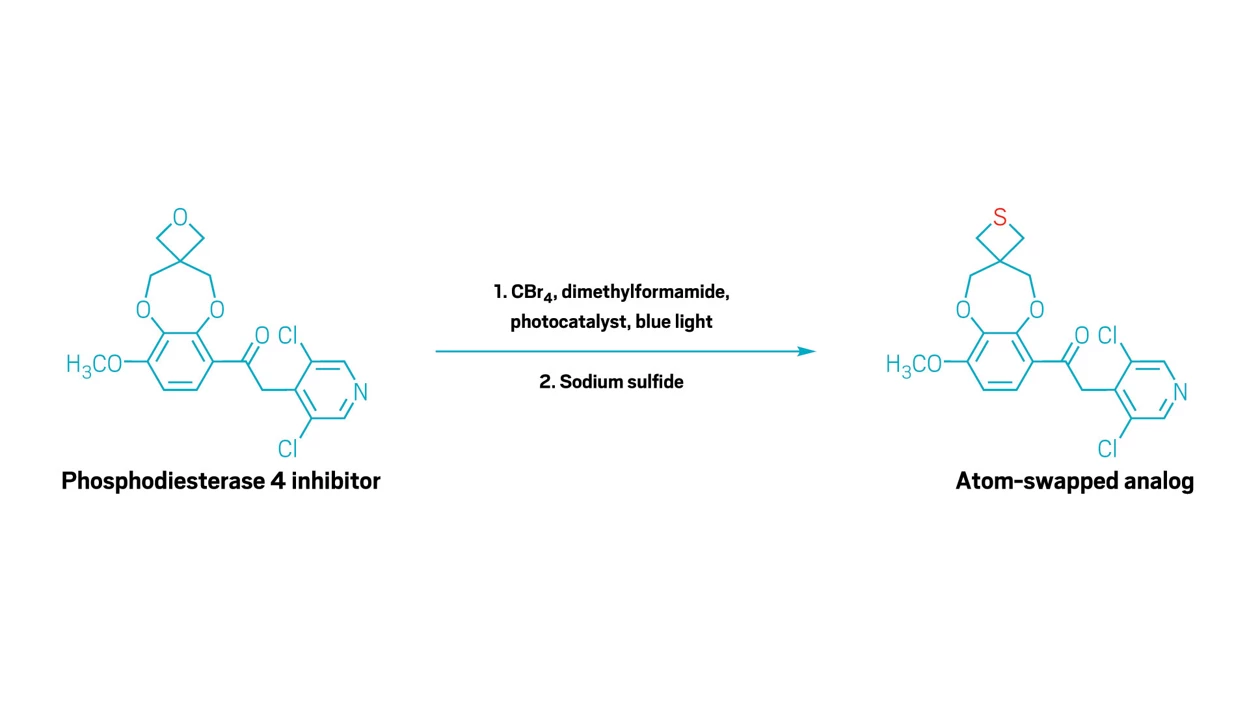
The team then react the partially deconstructed, brominated intermediate in a classic substitution reaction with a nucleophile to rebuild the ring around a different atom. Adding an amine and base, for example, creates an azetidine. Or adding sodium sulfide slides in a sulfur atom to give thietane rings—valuable pharmaceutical building blocks that are more challenging to make and much more expensive to buy than oxetane.
The researchers designed the reaction to play nicely with a variety of common organic functional groups, including other ethers. The starting material just can’t have any exposed hydroxy groups, because those will be susceptible to the bromination reaction.
To show off their reaction’s capabilities, the researchers used it to edit a variety of complex molecules, including turning an oxetane-containing anti-inflammatory molecule into a more potent thietane analog in a single operation. They also showed some examples in which starting from a commercially available oxetane and then swapping in a sulfur or nitrogen atom significantly cut the number of steps needed to create a key building block for a drug molecule.
Yoonsu Park of the Korea Advanced Institute of Science and Technology, who works on atom swapping but was not involved with the research, calls the paper “simply groundbreaking” work that “offers a powerful simplified logic” for constructing complex molecular fragments under mild conditions.
“We have these huge libraries of oxetanes . . . and the ability to kind of quickly diversify those, I think, will be really attractive,” says Zachary Wickens, an organic chemist at the University of Wisconsin–Madison who also was not involved with the work. “My guess is that people will try this almost right away.”
Brianna Barbu Brianna Barbu is a physical sciences reporter at C&EN. Bri is a DC-based science reporter covering organic chemistry and related topics. She also makes up 1/3 of C&EN’s structures team and is a regular contributor to the Chemistry in Pictures blog and Newscripts. Bri studied chemistry and creative writing at Hope College and earned a master’s degree in organic materials chemistry from the University of Michigan in 2019 before escaping the lab to pursue a career in journalism. Prior to joining C&EN, she was a 2021 AAAS Mass Media fellow at Discover magazine. When she’s not contemplating the weird world of carbon-based molecules, Bri enjoys swimming, crocheting, reading novels with her cat, and playing D&D.
Chemical & Engineering News
ISSN 0009-2347
Copyright ©
2025 American Chemical Society