Experimental location
This experiment was carried out from October 2023 to May 2024 in a commercial cattle farm located in the municipality of Rio Verde, Goiás, Brazil. This is a tropical region where there are two well-defined seasons per year: the rainy summer (October to April), with average annual rainfall of 1541 mm, and the dry winter (May to September), with 150–200 mm of rainfall.
The farm has about 4500 head of cattle. The cows are 1/2 Angus × 1/2 Nelore and the calves, obtained by artificial insemination, are 1/2 Angus × 1/2 Nelore and 3/4 European × 1/4 Nelore. Depending on their reproductive status, cows stay on the farm until their fifth pregnancy on average. All male calves and a portion of the female calves are confined after weaning and slaughtered at approximately 14 months of age. A portion of the 1/2 Angus × 1/2 Nelore female calves remain on the farm after weaning, on average, until the fifth gestation. The cattle on this farm are infested with R. microplus and maintain contact with TF pathogens. Sporadic outbreaks of A. marginale occur in calves between 17 and 25 days of age [6]. After 4 months of age, pasture-raised animals no longer present clinical problems due to TF pathogens, suggesting an enzootic stability to TF pathogens in animals from this age onwards.
For the past 12 years, cattle tick control on this farm has been based on empirical criteria established by farm staff, with treatment based on inspection of animals to visually assess the presence of ticks. The tick treatment used is a pour-on formulation of fipronil 1.25 mg/kg + fluazuron 2.5 mg/kg (TickGard®, MSD Animal Health). Pour-on fluralaner at 2.5 mg/kg (Exzolt® 5%, MSD Animal Health) had never been used on this farm before the start of this study.
Animals, groups, treatments, and experimental period
The study used 100 male and female 3/4 European × 1/4 Nelore calves that had not been treated for endo- or ectoparasites since birth and were naturally infested with R. microplus at the beginning of the study. When these animals were an average of 25 days old, they were divided into two groups of 50 animals each: FIFLUA and FLU. The FIFLUA group was treated with a commercial pour-on formulation of fipronil 1.25 mg/kg + fluazuron 2.5 mg/kg (TickGard®, MSD Animal Health) and the FLU group with a pour-on formulation of fluralaner 2.5 mg/kg (Exzolt® 5%, MSD Animal Health). The experimental period was based on the age of the calves. They were observed from 25 days of age until weaning, which occurred at an average age of 241 days. The criterion for allocating the animals to the groups was based on tick count/burden, age, and sex of the animals. After randomization, the groups were homogeneous in mean tick counts (FLU = 1.9 ± 2.5; FIFLUA = 1.8 ± 2.4), age in days (FLU = 25.3 ± 4.14; FIFLUA = 25.6 ± 5.24), and sex (FLU and FIFLUA = 23 females and 27 males each).
Throughout the experimental period (25 to 241 days of age), each group remained in a different paddock, with their respective mothers, practically identical in size and the availability of grass cover, and each group was kept separate from the other throughout the experimental period. The calves were fed maternal whole milk from cows during the entire period, plus Brachiaria brizantha and water ad libitum.
Strategic treatments against R. microplus on calves
The FIFLUA group served as a control. The commercial product used to treat the animals in this group was already in use on the farm. In addition, the animals receiving the treatment adopted by the farm no longer presented clinical cases of TF pathogens after approximately 2 to 4 months of age, suggesting enzootic stability for TF pathogens. The first treatment was performed on all animals of this group at 25 days of age. Therefore, the treatment criteria for the FIFLUA group corresponded to the practice of the farm staff. When this happened, all 50 animals in the group were treated.
The first treatment of the FLU group was performed at the beginning of the study (animals at 25 days of age). Calves in the FLU group were re-treated with fluralaner when more than 30% of the batch (15/50) had R. microplus infestations 13], independent of the number of ticks per animal. In each case, all 50 animals were treated. Visual inspection of animals in the FLU group was performed at weekly intervals throughout the experiment, always in the pasture.
The cows in each group received the same acaricide as the calves, but only once at the beginning of the study. In addition, the other activities described below did not apply to the cows.
Before each treatment in both groups, the animals were weighed to calculate the acaricide dose. The scales used to weigh the animals were previously tested with a known weight. The commercial products were administered topically using a 10-mL syringe graduated in 0.2-mL increments. Therefore, the calculated dose was rounded down; for example, an animal weighing 107 kg that would have received 10.7 mL of a pour-on product received 10.6 mL. Cattle treated with the pour-on formulations were not exposed to rain for 48 h after each treatment.
Tick counts and evaluation of the enzootic stability of TF
Female R. microplus ticks (between 4.5 and 8 mm in length) present on the left side of each animal (without multiplying by two) were counted on all calves [14] at the beginning of the study (25 days of age) to divide the animals into groups. Tick counts were then performed on both groups (FIFLUA and FLU) at average ages of 60, 135, 188, and 241 days (weaning).
The enzootic stability of the herd was assessed by indirect enzyme-linked immunosorbent assay (iELISA) on the same days as the tick counts, when the animals were 25, 60, 135, 188, and 241 days old. For this purpose, the serum or blood of 15 animals per group was randomly selected and evaluated throughout the experimental period. Immunoglobulin G (IgG) antibodies against A. marginale, B. bovis, and B. bigemina were detected by iELISA according to the protocol described by [15, 16].
Quantitative polymerase chain reaction (qPCR) was used to determine the frequency with which the animals of each group was exposed to the TF pathogens. To detect the DNA of the three agents, blood samples (300 µl) were subjected to DNA extraction using the DNAeasy Blood and Tissue Kit (Qiagen, Valencia, CA, USA), following the manufacturer’s instructions. The extracted DNA was tested using two different qPCR approaches: a monoplex qPCR assay targeting A. marginale and a duplex qPCR assay targeting B. bovis and B. bigemina. The monoplex qPCR assay targeted a fragment of the major surface protein 1b (msp1b) gene of A. marginale [17]. The duplex qPCR assay targeted the mitochondrial cytochrome B (cytB) gene of B. bovis and B. bigemina, as described by [18], with modifications to the quenchers (Table 1). Each qPCR run included a negative control (PCR-grade water, Sigma-Aldrich, St. Louis, MO, USA) and an appropriate positive control sample (DNA from A. marginale, B. bovis, or B. bigemina).
Table 1 Oligonucleotides (primers and probes) used in the present study
All qPCR assays were performed using TaqMan® Environmental Master Mix 2.0 (Applied Biosystems®, Foster City, CA, USA) according to the manufacturer’s recommendations, in a StepOnePlus™ Real-Time PCR System thermal cycler (Applied Biosystems®, Foster City, CA, USA). The thermal cycling conditions consisted of an initial DNA polymerase activation at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 s and annealing-extension at 60 °C for 1 min. For all assays, lower CT values corresponded to a higher amount of starting template, and a negative result was defined as having a CT value > 35 cycles. Negative samples were further tested using a conventional PCR protocol targeting a 359 bp fragment of the mammalian mitochondrial cytochrome B (cytB) protein gene [19] to validate the DNA extraction procedure. When a sample failed to produce any product in this PCR assay, it was excluded from further analysis. PCR products were stained with SYBR Safe (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s recommendations and visualized by 1.5% agarose gel electrophoresis under an ultraviolet transilluminator.
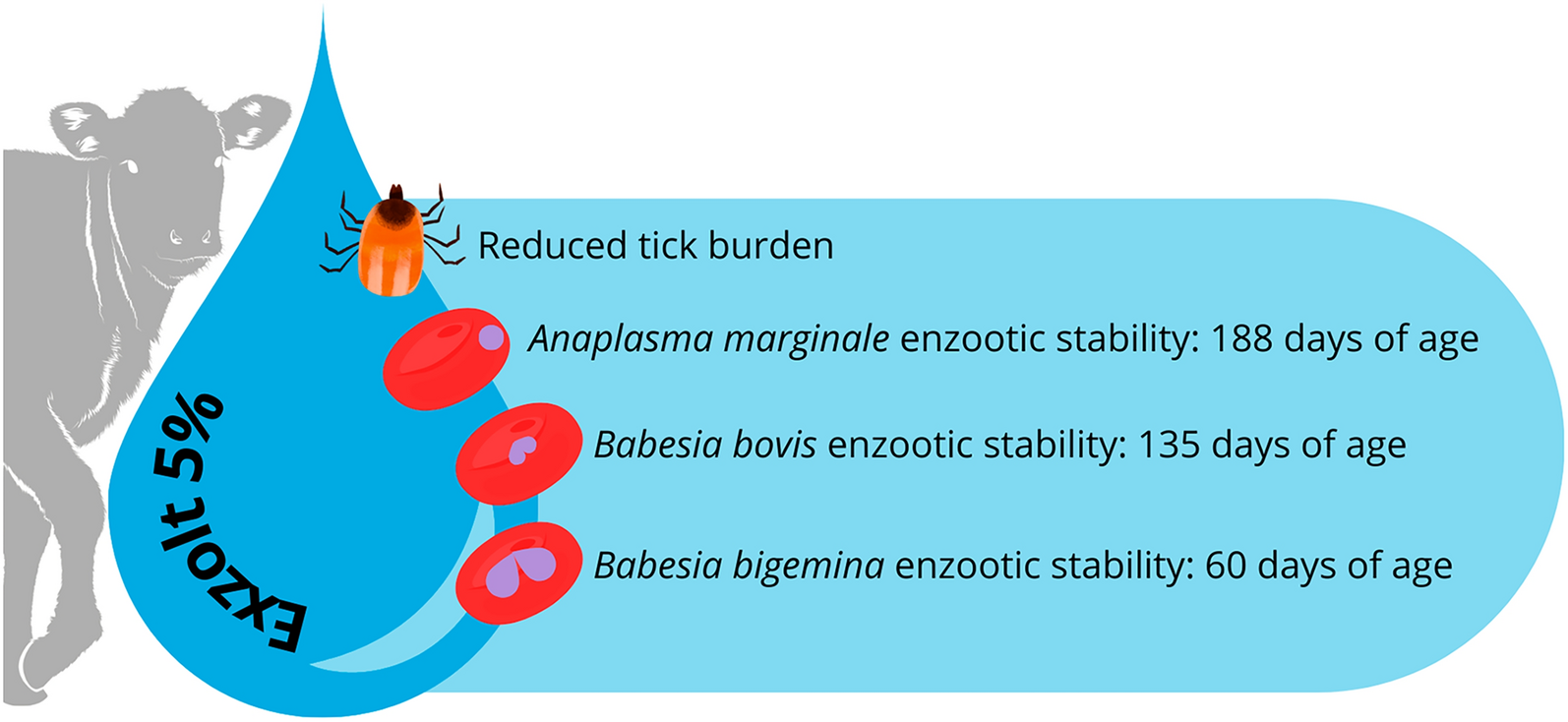
According to [8] and [9], a herd is considered enzootically stable for Babesia spp. and A. marginale if more than 75% of the animals are positive by serology for these pathogens. Therefore, in this study, enzootic stability was considered for each of the three TF agents when more than 75% of the animals in a group were positive by serology, while qPCR was used to confirm the frequency with which the animals of each group were exposed to the TF pathogens.
Blood smears: Babesia spp. parasitemia and A. marginale bacteremia
Blood smears were performed throughout the experiment to calculate Babesia spp. parasitemia and A. marginale bacteremia in the calves. However, this technique was not considered for the evaluation of enzootic stability due to its low sensitivity. Parasitological diagnosis was determined by blood smears for TF pathogens obtained from all animals in both groups at 25, 60, 135, 188, and 241 days of age. Blood was collected from the tip of the calves’ tails, stained with Giemsa, and examined under a microscope at 1000× magnification. The percentage of parasitemia or bacteremia for TF pathogens was determined using the method described by [20, 21].
Salvation treatment of the animals during the study against TF pathogens
To ensure animal welfare during the entire experimental period, if any calf showed clinical signs of TF pathogens, this animal received salvation treatment with diminazene 3.5 mg/kg (Ganazeg®, Elanco Animal Health [22]) plus 7.5 mg/kg of enrofloxacin intramuscularly (Knetomax®, Elanco Animal Health [23]). These signs involved the head, ears, and eyelids drooping, combined with the fact that the animal in question was more isolated from the herd, or that the animal was unable to keep up with the rest of the herd while the group was moving. Each time this happened, the animal’s identification and date were recorded, and a blood smear was performed to confirm the diagnosis of which TF agent was involved.
Statistical analyses
The data for tick counts and serological tests did not meet the assumptions of normality, homogeneity of variance, residuals, and randomness, even after transforming the data into log(count + 1). Therefore, the experimental groups were compared using the Kruskal–Wallis test.
All statistical procedures were performed using the Statistical Analysis System [24], version 9.4 (2016). Differences were considered statistically significant when P