Curcumin, a dietary polyphenol derived from Curcuma longa, has been extensively studied for its pleiotropic biological activities, particularly its anticancer potential. This systematic review provides compelling preclinical evidence supporting curcumin’s multi-targeted effects in prostate cancer (PCa), acting through diverse molecular pathways, cellular mechanisms, and delivery strategies. The findings highlight both the therapeutic promise and translational challenges associated with curcumin-based interventions.
While numerous reviews have summarized the general anticancer properties of curcumin, our work is novel in that it systematically and exclusively focuses on prostate cancer, incorporating both molecular mechanisms and nanoformulation strategies. By applying rigorous quality assessment tools and including studies published up to March 2025, this review provides an updated and more clinically oriented perspective compared to earlier narrative or broad-spectrum reviews.
A prominent mechanism by which curcumin exerts antitumor activity is through inhibition of the PI3K/Akt/mTOR axis, a signaling hub critically involved in prostate tumorigenesis, particularly in PTEN-deficient contexts [11]. Notably, nanoparticle-formulated curcumin (Theracurmin) effectively downregulated PI3K/Akt signaling in early-stage PCa without inducing apoptosis, suggesting a chemopreventive rather than cytotoxic mode of action [11]. In contrast, functionalized hybrid nanoparticles co-delivering curcumin and cabazitaxel not only suppressed PI3K/Akt and NF-κB pathways but also synergistically enhanced apoptosis and tumor regression in vivo, demonstrating the power of rational combination therapy [12].
Curcumin’s modulation of androgen receptor (AR) signaling further underpins its relevance in both hormone-sensitive and castration-resistant PCa. The curcumin analog H10 selectively inhibited 17β-HSD3 activity, reducing intratumoral testosterone levels without affecting upstream enzymes [27]. This targeted steroidogenesis inhibition translated to AR suppression and tumor growth reduction. Similarly, combined curcumin-dutasteride treatment significantly reduced PSA expression, induced DNA damage, and enhanced apoptosis, reinforcing curcumin’s potential as an adjunct to androgen deprivation therapy [14].
Apoptosis induction was a consistent and potent effect observed across multiple models. Mechanistic studies revealed activation of intrinsic (Bax, caspase-3) and extrinsic (DR4/DR5) apoptotic pathways, often coupled with mitochondrial dysfunction and ROS generation [13, 15, 17]. Interestingly, in acidity-adapted PCa cells mimicking the tumor microenvironment, curcumin triggered both apoptosis and necroptosis—a rare and highly cytotoxic combination—via oxidative stress and ATP depletion, selectively killing cancer cells while sparing normal epithelium [15]. Such dual-mode cell death could offer a promising strategy to overcome resistance in advanced PCa.
Beyond cell death, curcumin inhibited migration, invasion, and angiogenesis by modulating EMT markers (e.g., E-cadherin↑, ZEB1↓, vimentin↓) and angiogenic regulators (CD31↓) [16, 20]. These effects are crucial in metastatic PCa models such as PC-3 and DU145, suggesting curcumin’s utility not only in tumor suppression but also in preventing disease progression.
However, curcumin’s poor bioavailability remains a major translational barrier [9]. Several studies overcame this by employing nanoformulations—Theracurmin, dextran-curcumin systems, scorpion venom-conjugated phytosomes, and PLGA nanoparticles—each showing enhanced solubility, cellular uptake, and antitumor efficacy [11, 13, 16, 22]. Notably, PLGA nanoparticles co-loaded with curcumin and piperine inhibited steroid biosynthesis and androgen production in PCa cell lines more effectively than free curcumin [22]. Such delivery platforms not only improve pharmacokinetics but also enable lower dosing and targeted delivery.
The synergistic effects of curcumin in combination therapies are another major highlight. Co-administration with chemotherapeutics (e.g., docetaxel, cisplatin), natural compounds (e.g., quercetin, carnosic acid), or physical enhancers (e.g., light irradiation) consistently amplified its anticancer potency [19, 24, 25]. For instance, light-activated curcumin suppressed proliferation and integrin signaling at ultralow doses, with minimal toxicity, opening avenues for photodynamic interventions [19]. These findings emphasize that curcumin’s full therapeutic potential may be realized when integrated into multimodal treatment regimens.
Mechanistically, curcumin’s interaction with emerging molecular targets adds a novel dimension to its anticancer action. Several studies reported upregulation of tumor-suppressive microRNAs (miR-30a-5p, miR-383, miR-708), leading to inhibition of key oncogenic proteins like PCLAF, LDHA, and RAP1B [17, 31]. Other studies demonstrated copper-mediated redox cycling and oxidative DNA damage induced by curcumin in cancer cells, offering a mechanism of selective cytotoxicity not reliant on canonical apoptotic triggers [29]. Furthermore, modulation of energy and lipid metabolism through direct binding to PDHA1 and downregulation of lipogenic genes suggests a metabolic reprogramming role for curcumin in PCa [21].
While previous reviews have addressed the anticancer effects of curcumin in general oncology, few have provided a systematic and mechanistic synthesis focused specifically on prostate cancer. Furthermore, most earlier reviews lacked critical appraisal of study quality and did not comprehensively analyze advanced delivery systems. This review is distinct in integrating molecular mechanistic insights with nanoformulation strategies, offering a more translational perspective. The inclusion of recent studies (up to March 1, 2025) ensures that the findings are current and reflective of evolving research trends.
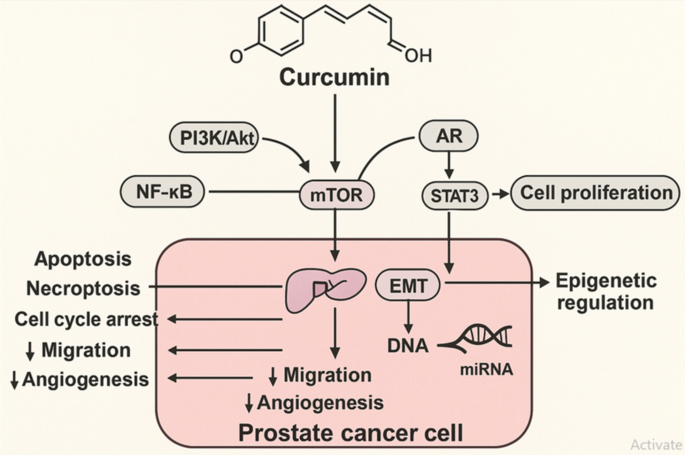
Figure 4 summarizes the principal molecular pathways modulated by curcumin in prostate cancer models, highlighting its inhibitory effects on oncogenic signaling, epigenetic regulators, and downstream hallmarks of cancer progression.
Mechanistic pathways targeted by curcumin in prostate cancer. Curcumin modulates multiple oncogenic signaling pathways—including PI3K/Akt/mTOR, NF-κB, androgen receptor (AR), and STAT3—leading to suppression of cell proliferation, induction of apoptosis and necroptosis, cell cycle arrest, and inhibition of migration and angiogenesis. Curcumin also regulates epigenetic mechanisms via miRNA modulation and EMT inhibition, contributing to reduced tumor aggressiveness and metastatic potential. This figure also serves as a graphical summary of the study, highlighting the dual focus on molecular mechanisms and nanoformulation strategies of curcumin in prostate cancer
Limitations and future directions
Despite the promising preclinical evidence, several limitations must be acknowledged. The heterogeneity of curcumin formulations, dosing regimens, and treatment durations across studies impedes direct comparison and prevents the definition of clear efficacy thresholds. Moreover, variability in experimental models—including differences between prostate cancer cell lines and diverse in vivo systems (e.g., xenograft vs. transgenic models)—further complicates the extrapolation of findings.
Another important consideration is the potential for publication bias. Studies reporting positive results are more likely to be published, which could lead to an overestimation of curcumin’s true therapeutic potential in prostate cancer models. A systematic effort to include unpublished negative or neutral data would provide a more balanced perspective.
Beyond issues of bioavailability, translational challenges associated with nanoformulations also warrant attention. These include the risk of unforeseen toxicity or immunogenic responses, difficulties in large-scale manufacturing, and batch-to-batch variability that can undermine reproducibility and regulatory approval. Such hurdles must be systematically addressed before curcumin nanoformulations can advance into late-stage clinical evaluation.
Future research should prioritize: (1) standardized dosing and formulation protocols; (2) pharmacokinetic and toxicological profiling in both animals and early-phase clinical studies; (3) biomarker-driven patient stratification for personalized curcumin-based therapy; and (4) exploration of curcumin as an adjuvant in immunotherapy and radiotherapy contexts.