Dublin, Oct. 21, 2025 (GLOBE NEWSWIRE) — The “Viral Vector Manufacturing Markets and Technologies Through 2030” report has been added to ResearchAndMarkets.com’s offering.
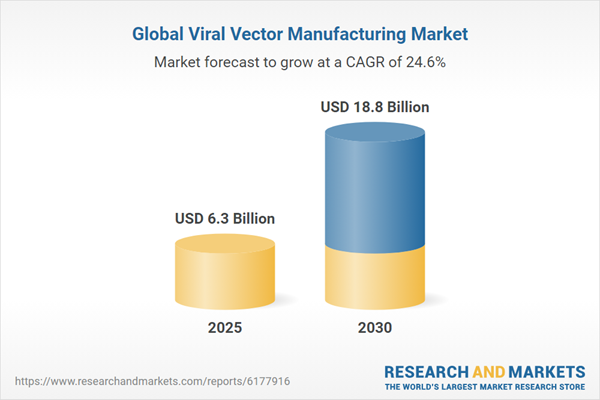
The global market for viral vector manufacturing is expected to grow from $6.3 billion in 2025 and is projected to reach $18.8 billion by the end of 2030, at a compound annual growth rate (CAGR) of 24.6% during the forecast period of 2025 to 2030.
The use of viral vectors in gene therapy is transforming healthcare, with an increase in the demand for Good Manufacturing Practice (GMP) grade materials. The process of bringing a viral vector from concept to clinic requires extensive optimization and characterization, affecting every stage from cell culture to purification and concentration. Variations in vector titer during the upstream phase may result from the specific transgene being packaged or from constitutive or “leaky” tissue-specific promoters, which can induce unintended protein expression, and, in turn, impact vector yield and quality. Therefore, increasing the production of GMP-grade materials is crucial to meet the growing demand for clinical applications.
Market Dynamics and Growth Factors
Viral-based vectors are at the core of cell and gene therapy, enabling researchers and clinicians to develop powerful drug platforms. Over the past five years, regulatory approvals for viral vector-based drugs have surged, ranging from cancer therapies to treatments for monogenic diseases. The three dominant vector strategies are adenoviruses, adeno-associated viruses, and lentiviruses, which have led to preclinical and clinical breakthroughs over the last two decades.
Market drivers include the increasing incidence of chronic diseases, rising approvals of vector-based therapies, and growing demand for viral vector production. Market restraints include the process and cost barriers in viral vector development, roadblocks with viral vectors, and the high cost linked with viral vector production.
Report Scope
This report analyzes viral vector manufacturing market trends using 2024 data, 2025 estimates, market projections and compound annual growth rates (CAGRs) for the forecast period of 2025-2030. The report discusses viral vector manufacturing’s current and future market potential and covers regulatory scenarios, drivers, restraints, and opportunities.
The report segments the market by type, application and disease. Types include adenoviral vectors (AdVs), adeno-associated viral vectors (AAVs), lentiviral vectors (LVs), retroviral vectors (RVs), and other viral vectors. Applications consist of multivalent and multipathogen. Diseases are segmented into human and veterinary. Human diseases are further segmented into HIV, tuberculosis, cancer, influenza, malaria, hepatitis, and others. Veterinary diseases are further segmented into avian influenza, Marek’s disease, infectious bronchitis, and PPR disease.
The goals of the report are to:
- Analyze types of viral vectors.
- Calculate the size of the global market size, with forecasts through 2030.
- Identify market constraints and drivers.
- Assess company’s market shares, competitiveness, and industry structure.
- Identify potential long-term impacts on the viral vector manufacturing market.
Report Features
- Overview and analysis of the global viral vector manufacturing markets and technologies
- Analyses of the global market trends, with historic sales revenue data from 2022 to 2024, estimates for 2025, and projected CAGRs through 2030
- Estimates of the market’s size and revenue prospects, accompanied by a market share analysis based on type, application, disease segment and region
- Facts and figures pertaining to market dynamics, technological advancements, regulations, prospects, and the impacts of macroeconomic variables
- Insights derived from Porter’s Five Forces model and global value chain analysis
- An assessment of the viral vaccine pipeline products, clinical trials, competitive scenario and R&D activities
- An analysis of patents, emerging trends and new developments in the industry
- Overview of sustainability trends and ESG developments, with emphasis on consumer attitudes, and the ESG scores and practices of leading companies
- Analysis of the industry structure, including companies’ market shares and rankings, strategic alliances, and M&A activity
- Profiles of the leading companies
- ABL Inc.
- Catalent Inc.
- Charles River Laboratories
- Danaher Corp.
- F. Hoffmann-La Roche Ltd.
- Fujifilm Diosynth Biotechnologies
- Lonza
- Merck Kgaa
- Novartis Ag
- Oxford Biomedica Plc.
- Probio
- Revvity
- Sanofi
- Takara Bio Inc.
- Thermo Fisher Scientific Inc.
Key Attributes:
Key Topics Covered:
Chapter 1 Executive Summary
- Market Outlook
- Scope of Report
- Market Summary
- Market Dynamics and Growth Factors
- Emerging Technologies
- Segmental Analysis
- Regional Analysis
Chapter 2 Market Overview
- Macroeconomic Factors
- Impact of U.S. Tariffs on the Pharmaceutical Industry
- Healthcare Expenditures
- Impact of Global Economic Growth (GDP) on Pharma Sector
- Direct Economic Impact
- Indirect Economic Impact
- Porter’s Five Forces Analysis
Chapter 3 Market Dynamics
- Market Drivers
- Increasing Incidence of Chronic Diseases
- Approvals of Vector-Based Therapies
- Demand for Viral Vector Production
- Market Restraints
- Process and Cost Barriers in Viral Vector Development
- Viral Vector Roadblocks
- High Cost Linked with Viral Vector Production
- Market Opportunities
- Emerging Viral-Based Therapies
- Digitalization in Viral Vector Production
Chapter 4 Regulatory Landscape
Chapter 5 Emerging Technologies and Pipeline Analysis
- Automation
- Next-Generation Production Systems
- Genome Editing
- Pipeline Analysis
- Takeaways
- Patent Analysis
- Takeaways
Chapter 6 Market Segmentation Analysis
- Global Market for Viral Vector Manufacturing by Type
- Lentiviral Vectors
- Adenoviral Vectors
- Adeno-Associated Viral Vectors
- Retroviral Vectors (RVs)
- Other Viral Vectors
- Global Viral Vector Manufacturing Market, by Application
- Multivalent Viral Vector Vaccines
- Multipathogen Viral Vector Vaccines
- Global Market for Viral Vector Manufacturing, by Disease
- Veterinary Diseases
- Human Diseases
- Global Market for Viral Vector Manufacturing, by Region
- North America
- Europe
- Asia-Pacific
- Middle East and Africa
- South America
Chapter 7 Competitive Intelligence
- Market Share Analysis
- Market Share Analysis
- Recent Developments
Chapter 8 Sustainability in the Viral Vector Manufacturing Industry: An ESG Perspective
- Introduction to ESG
- Sustainability in the Viral Vector Manufacturing Industry
- ESG Perspective
- Environmental Impact
- Waste Management
- Social Impact
- Governance Impact
- ESG Risk Ratings
Company Profiles
- ABL Inc.
- Catalent Inc.
- Charles River Laboratories
- Danaher Corp.
- F. Hoffmann-La Roche Ltd.
- Fujifilm Diosynth Biotechnologies
- Lonza
- Merck Kgaa
- Novartis Ag
- Oxford Biomedica Plc.
- Probio
- Revvity
- Sanofi
- Takara Bio Inc.
- Thermo Fisher Scientific Inc.
For more information about this report visit https://www.researchandmarkets.com/r/2tlsv2
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world’s leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.

