Peripheral venous catheter (PVC) insertion, a routine procedure performed in over 80% of hospitalized patients [8], is not without risks. Complications, including clotting, occlusion, leakage, infiltration, extravasation, phlebitis, and infection, present challenges in clinical management [9]. Pediatric departments report a relatively lower incidence of complications, approximately a quarter of PVC cases [10].
While complications associated with PVCs are generally minor, our case report underscores the importance of comprehending and identifying severe complications, such as extravasation. Extravasation, the leakage of intravenous fluid into surrounding tissue, is an infrequent yet potentially serious complication requiring immediate attention. The initial suspicion of extravasation prompted the immediate removal of the intravenous catheter and the initiation of appropriate interventions. This rapid response was critical in minimizing systemic deterioration and highlights the essential role of early recognition and timely escalation.
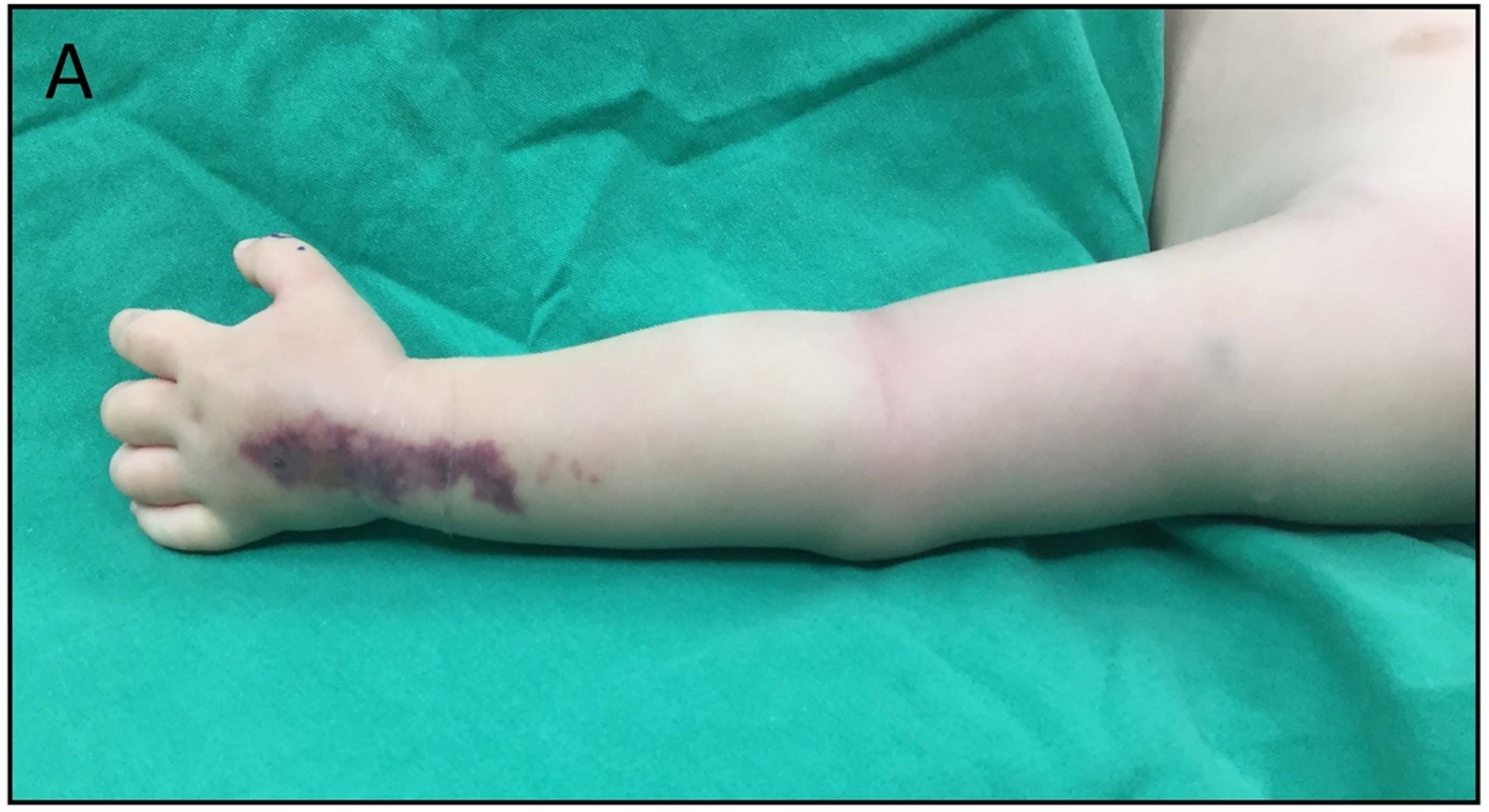
Extravasation manifests with characteristic signs and symptoms, including swelling and erythema at the catheter insertion site. Our patient displayed a rapid progression of symptoms from the hand to the upper extremity. In pediatric patients, distinguishing between early signs of extravasation and emerging infection is uniquely challenging due to communication limitations and often subtle clinical signs. This reinforces the need for heightened surveillance protocols. Our institutional protocol for peripheral venous catheter (PVC) management mandates daily visual inspection, documentation of insertion site condition, and replacement every 72–96 h. A standardized nursing checklist records signs of phlebitis, extravasation, or infection every shift. The documentation sheet includes fields for insertion date/time, catheter gauge, skin preparation agent used, and site assessment scoring.
The differential diagnosis of PVC-related complications encompasses extravasation, phlebitis, cellulitis, suppurative thrombophlebitis, and, as observed in our patient, necrotizing soft tissue infection (NSTI). Each requires a distinct approach, and misdiagnosis could lead to delayed or inappropriate management. While our patient lacked typical NSTI risk factors such as trauma, surgery, or burn injury, the development of NSTI in the context of a routine PVC highlights the diagnostic challenge posed by subtle presentations in low-risk children. This case emphasizes the need for clinical vigilance, even in seemingly benign settings. In this context, our report may serve as a reference point for the incorporation of NSTI into differential diagnoses for rapidly progressing soft tissue changes near PVC sites, even in the absence of systemic signs.
Routine replacement of PVCs has been advocated to mitigate the risks of phlebitis and bloodstream infections. Recent reviews, such as Chen’s study in 2022, suggest that routine replacement every 72–96 h may be a preferred option, minimizing the risks of phlebitis, infiltration, and occlusion [11]. In contrast, a 2021 randomized controlled trial by Lin et al. in pediatric patients demonstrated that clinically indicated peripheral intravenous replacement (CIPIR) significantly prolonged catheter dwell time without increasing rates of infiltration, occlusion, or phlebitis [12]. These findings suggest that in children, replacing PVCs based on clinical signs rather than a fixed schedule may be both safe and effective. In our case, although the complication occurred on day 3, our practice of routine site evaluation and clinically indicated replacement aligns with the US Centers for Disease Control guidelines [13].
The skin preparation protocol before PVC insertion plays a crucial role in preventing complications. Although our institution currently employs iodine-alcohol, aligning with CDC guidelines, the potential improvement with the use of chlorhexidine 2% in combination with alcohol 70% is recognized. International guidelines confirm the superiority of chlorhexidine solution in reducing bacterial contamination on devices [14]. Skin preparation protocols for PVC insertion must be tailored to pediatric patients. A recent study in neonates showed that 2% chlorhexidine in 70% isopropyl alcohol (CHG-IPA) achieved superior bacterial reduction without compromising skin integrity, compared to povidone-iodine and alcohol alone [15]. These findings emphasize the need for pediatric-specific antiseptic strategies rather than direct application of adult protocols.
This case suggests that the development of necrotizing soft tissue infection (NSTI) may be linked to a late diagnosis of extravasation [16], possibly attributed to inadequate monitoring. Our findings also draw attention to systemic staffing issues. In our center, nurse-to-patient ratios ranged from 1:13 to 1:20 during night shifts, potentially contributing to delayed recognition of catheter-related complications. Previous studies have demonstrated that lower nurse-to-patient ratios are directly associated with improved detection of complications and decreased rates of adverse outcomes [17]. We advocate for maintaining a ratio of 1:6 or better in pediatric wards, particularly where high-risk interventions such as PVCs are performed.
The challenges identified in routine replacement practices, skin preparation protocols, and monitoring underscore the need for continuous improvement in clinical practices. To enhance patient safety, hospitals should consider adopting evidence-based recommendations, such as routine replacement strategies and optimal skin preparation solutions. Furthermore, addressing staffing issues to ensure adequate nurse-to-patient ratios is imperative for timely identification and management of complications associated with PVCs.
The historical approach to necrotizing fasciitis involved aggressive skin excision, extending at least as wide as the cellulitis [18]. Wong et al.‘s approach introduced a four-step method emphasizing confirmation of diagnosis, identifying causative organisms, determining fascial extent, and excising up to tissue with normal vascularity [19]. The current surgical approach, as employed in our patient, adopts a more tailored strategy, restricting skin excision to the area of definite skin necrosis. The incision aligns with relaxed skin tension lines for optimal exposure and fascial debridement. Debridement extends into healthy-appearing tissue, confirmed by frozen sections. Adjuncts such as negative-pressure wound therapy and hyperbaric oxygen therapy are utilized as needed. Second-look operations, performed after 24 h, address any residual necrosis before attempted closure.
Compared to traditional wide excisions, tissue-sparing debridement offers the advantages of reduced aesthetic and functional morbidity, shorter hospital stays, and faster rehabilitation—particularly vital in pediatric patients. This technique also has favorable cost-effectiveness due to preservation of viable tissue and reduction in reconstructive needs. However, it requires timely surgical access, reliable imaging, and intraoperative judgment, which may not be universally accessible in all settings.
The definition of “adequate” debridement remains subjective, involving the removal of all necrotic tissue and left to the discretion of the operating surgeon. The aggressiveness of debridement, often described as “aggressive,” lacks clear quantification. Reevaluation in the operating room within 24–48 h is common, aiming to re-evaluate the wound, debride further necrotic tissue, confirm the absence of progression, and facilitate dressing changes [20].
Historical practices at our institution favored aggressive surgical management, involving excision of the skin overlying necrotic soft tissues. The current goal of surgical intervention is the removal of infected and nonviable tissues, including skin, fascia, and muscle, aiming to control the progression of necrotizing fasciitis and limit the diffusion of bacterial toxins [21]. While imaging, including ultrasonography and MRI, serves the purpose of confirming deep tissue involvement, the gold standard for diagnosing necrotizing fasciitis remains surgical exploration with frozen section analysis. Our tailored surgical intervention, involving tissue-sparing debridement, represents a departure from historical aggressive approaches. The objective is to achieve effective control of the disease while minimizing unnecessary excision, particularly in pediatric patients. The concept of “aggressive” debridement, while common, lacks precise quantification, and ongoing reevaluation in the operating room remains essential for optimal wound management.
In pediatric patients, the advantages of tissue-sparing debridement become particularly evident, ensuring functional and aesthetic outcomes. The approach balances the need for thorough infection control with minimizing unnecessary tissue loss, acknowledging the unique challenges posed by the pediatric population [21]. Further research should focus on refining debridement criteria, quantifying the balance between tissue preservation and infection control. Pediatric-specific guidelines for necrotizing fasciitis management can contribute to improved outcomes and reduced morbidity in this vulnerable population. Continuous collaboration between surgical teams and plastic surgeons is crucial for evolving best practices in pediatric necrotizing fasciitis.
Monotherapy agents, including imipenem, meropenem, ertapenem, piperacillin/tazobactam, and tigecycline, can be employed. Multidrug regimens, such as triple-drug therapy, may be considered. Agents like vancomycin, daptomycin, or linezolid should be included until methicillin-resistant staphylococcal infection is ruled out. Protein synthesis inhibitors like clindamycin, which can inhibit toxin production, play a crucial role in controlling the inflammatory response, particularly in clostridial and streptococcal infections [22]. However, broad-spectrum antimicrobial therapy is not without risk. Overuse may lead to the emergence of multidrug-resistant organisms, secondary infections, and unnecessary toxicity. Infectious diseases (ID) specialist consultation is critical in guiding early de-escalation and ensuring antibiotic stewardship. Local epidemiologic data—such as MRSA prevalence and rates of carbapenem-resistant Enterobacteriaceae (CRE) or extended-spectrum beta-lactamase (ESBL)-producing organisms—should inform empiric antibiotic selection. In our institution, MRSA prevalence remains significant, justifying initial vancomycin coverage, while CRE and ESBL incidence remains low. Antimicrobial therapy should be administered until no further debridements are needed and continued until the patient’s physiology improves. Prolonged courses of arbitrary duration are discouraged to prevent colonization with drug-resistant organisms.
Source control, achieved through early and complete debridement, remains the cornerstone of NSTI treatment. Antimicrobial therapy, providing broad-spectrum coverage and considering specific pathogens, serves as a crucial adjunct to source control. Early and aggressive antibiotic therapy is crucial and should be initiated concomitantly with surgical evaluation and treatment. Prompt blood cultures and deep tissue, abscess, or operative cultures aid in tailoring antibiotic therapy based on culture results. Empiric therapy should encompass broad-spectrum coverage, considering the polymicrobial nature of infections [23]. This includes an MRSA-active agent and a broad-spectrum agent against gram-negative pathogens, along with anaerobic activity. Considerations such as the use of tigecycline in patients previously colonized with resistant bacteria and avoiding empiric use of certain agents in areas with high resistance prevalence are crucial [24]. While not essential empirically, antifungal therapy may be added upon evidence of fungal elements in cultures. Tailoring therapy based on culture results, with consideration for discontinuation of MRSA-active agents guided by negative predictive value, is emphasized. The duration of therapy, not extensively studied, is suggested to continue for a minimum of 48–72 h after resolution of fever, systemic signs, and hemodynamic stabilization. IDSA practice guidelines for skin and soft tissue infections provide additional details on antibiotic therapy for NSTI [23].