Ulaeto D, Agafonov A, Burchfield J, Carter L, Happi C, Jakob R, et al. New nomenclature for mpox (monkeypox) and monkeypox virus clades. Lancet Infect Dis. 2023;23(3):273–5.
Li H, Zhang H, Ding K, Wang XH, Sun GY, Liu ZX, et al. The evolving epidemiology of monkeypox virus. Cytokine Growth Factor Rev. 2022;68:1–12.
Farasani A. Monkeypox virus: future role in human population. J Infect Public Health. 2022;15(11):1270–5.
Otieno JR, Ruis C, Onoja AB, Kuppalli K, Hoxha A, Nitsche A, et al. Global genomic surveillance of monkeypox virus. Nat Med. 2025;31(1):342–50.
Gigante CM, Korber B, Seabolt MH, Wilkins K, Davidson W, Rao AK, et al. Multiple lineages of monkeypox virus detected in the United States, 2021–2022. Science. 2022;378(6619):560–5.
Ndodo N, Ashcroft J, Lewandowski K, Yinka-Ogunleye A, Chukwu C, Ahmad A, et al. Distinct monkeypox virus lineages co-circulating in humans before 2022. Nat Med. 2023;29(9):2317–24.
Isidro J, Borges V, Pinto M, Sobral D, Santos JD, Nunes A, et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat Med. 2022;28(8):1569–72.
Scarpa F, Sanna D, Azzena I, Cossu P, Locci C, Angeletti S, et al. Genetic variability of the monkeypox virus clade IIb B.1. J Clin Med. 2022;11(21):6388.
Nuzzo JB, Borio LL, Gostin LO. The WHO declaration of monkeypox as a global public health emergency. JAMA. 2022;328(7):615–7.
Gessain A, Nakoune E, Yazdanpanah Y. Monkeypox. N Engl J Med. 2022;387(19):1783–93.
Wawina-Bokalanga T, Akil-Bandali P, Kinganda-Lusamaki E, Lokilo E, Jansen D, Amuri-Aziza A, et al. Co-circulation of monkeypox virus subclades Ia and Ib in Kinshasa Province, democratic republic of the Congo, July to August 2024. Euro Surveill. 2024;29(38):2400592.
Eurosurveillance Editorial Team. Note from the editors: WHO declares mpox outbreak a public health emergency of international concern. Euro Surveill. 2024;29(33):240815v.
WHO director-general declares mpox outbreak a public health emergency of international concern. Saudi Med J. 2024;45(9):1002–03.
Zhang Z, Jiang H, Jiang S, Dong T, Wang X, Wang Y, et al. Rapid detection of the monkeypox virus genome and antigen proteins based on surface-enhanced Raman spectroscopy. ACS Appl Mater Interfaces. 2023;15(29):34419–26.
Huo S, Chen Y, Lu R, Zhang Z, Zhang G, Zhao L, et al. Development of 2 multiplex real-time PCR assays for simultaneous detection and differentiation of monkeypox virus IIa, IIb, and I clades and the B.1 lineage. Biosaf Health. 2022;4(6):392–8.
Lu T, Wu Z, Jiang S, Lu L, Liu H. The current emergence of monkeypox: The recurrence of another smallpox? Biosaf Health. 2022;4(6):369–75.
Lim CK, Roberts J, Moso M, Liew KC, Taouk ML, Williams E, et al. Monkeypox diagnostics: review of current and emerging technologies. J Med Virol. 2023;95(1):e28429.
McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis. 2014;58(2):260–7.
Fine PE, Jezek Z, Grab B, Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17(3):643–50.
Gruber MF. Current status of monkeypox vaccines. NPJ Vaccines. 2022;7(1):94.
Kandeel M, Morsy MA, Abd El-Lateef HM, Marzok M, El-Beltagi HS, Al Khodair KM, et al. Efficacy of the modified vaccinia Ankara virus vaccine and the replication-competent vaccine ACAM2000 in monkeypox prevention. Int Immunopharmacol. 2023;119:110206.
Hazra A, Rusie L, Hedberg T, Schneider JA. Human monkeypox virus infection in the immediate period after receiving modified vaccinia ankara vaccine. JAMA. 2022;328(20):2064–7.
Sang Y, Zhang Z, Liu F, Lu H, Yu C, Sun H, et al. Monkeypox virus quadrivalent mRNA vaccine induces immune response and protects against vaccinia virus. Signal Transduct Target Ther. 2023;8(1):172.
Zaeck LM, Lamers MM, Verstrepen BE, Bestebroer TM, van Royen ME, Götz H, et al. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat Med. 2023;29(1):270–8.
Rizk JG, Lippi G, Henry BM, Forthal DN, Rizk Y. Prevention and treatment of monkeypox. Drugs. 2022;82(9):957–63.
Huston J, Curtis S, Egelund EF. Brincidofovir: a novel agent for the treatment of smallpox. Ann Pharmacother. 2023;57(10):1198–206.
Hoy SM. Tecovirimat: first global approval. Drugs. 2018;78(13):1377–82.
Alakunle E, Kolawole D, Diaz-Cánova D, Alele F, Adegboye O, Moens U, et al. A comprehensive review of monkeypox virus and monkeypox characteristics. Front Cell Infect Microbiol. 2024;14:1360586.
Lim EY, Whitehorn J, Rivett L. Monkeypox: a review of the 2022 outbreak. Br Med Bull. 2023;145(1):17–29.
Wang L, Shang J, Weng S, Aliyari SR, Ji C, Cheng G, et al. Genomic annotation and molecular evolution of monkeypox virus outbreak in 2022. J Med Virol. 2023;95(1):e28036.
Smith TG, Gigante CM, Wynn NT, Matheny A, Davidson W, Yang Y, et al. Tecovirimat resistance in monkeypox patients, United States, 2022–2023. Emerg Infect Dis. 2023;29(12):2426–32.
Mitjà O, Alemany A, Marks M, Lezama Mora JI, Rodríguez-Aldama JC, Torres Silva MS, et al. monkeypox in people with advanced HIV infection: a global case series. Lancet. 2023;401(10380):939–49.
Andrei G, Snoeck R. Differences in pathogenicity among the monkeypox virus clades: impact on drug discovery and vaccine development. Trends Pharmacol Sci. 2023;44(10):719–39.
Zhang Y, Zhang JY, Wang FS. Monkeypox outbreak: a novel threat after COVID-19?. Mil Med Res. 2022;9(1):29.
Denkinger CM, Janssen M, Schäkel U, Gall J, Leo A, Stelmach P, et al. Anti-SARS-CoV-2 antibody-containing plasma improves outcome in patients with hematologic or solid cancer and severe COVID-19: a randomized clinical trial. Nat Cancer. 2023;4(1):96–107.
Marconato M, Abela IA, Hauser A, Schwarzmüller M, Katzensteiner R, Braun DL, et al. Antibodies from convalescent plasma promote SARS-CoV-2 clearance in individuals with and without endogenous antibody response. J Clin Invest. 2022;132(12):e158190.
Klasse PJ, Moore JP. Antibodies to SARS-CoV-2 and their potential for therapeutic passive immunization. Elife. 2020;9:e57877.
Caskey M, Klein F, Nussenzweig MC. Broadly neutralizing anti-HIV-1 monoclonal antibodies in the clinic. Nat Med. 2019;25(4):547–53.
Li M, Wang Y, Li C, Xu R, Chen J, Zhang J, et al. Development of monoclonal antibody-based antigen detection assays for orthopoxvirus and monkeypox virus. J Infect. 2022;85(6):702–69.
Zuiani A, Dulberger CL, De Silva NS, Marquette M, Lu YJ, Palowitch GM, et al. A multivalent mRNA monkeypox virus vaccine (BNT166) protects mice and macaques from orthopoxvirus disease. Cell. 2024;187(6):1363–73.e12.
Lederman ER, Davidson W, Groff HL, Smith SK, Warkentien T, Li Y, et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis. 2012;206(9):1372–85.
Lu J, Xing H, Wang C, Tang M, Wu C, Ye F, et al. Monkeypox (formerly monkeypox): pathogenesis, prevention, and treatment. Signal Transduct Target Ther. 2023;8(1):458.
Shi D, He P, Song Y, Cheng S, Linhardt RJ, Dordick JS, et al. Kinetic and structural aspects of glycosaminoglycan-monkeypox virus protein A29 interactions using surface plasmon resonance. Molecules. 2022;27(18):5898.
Lin CL, Chung CS, Heine HG, Chang W. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J Virol. 2000;74(7):3353–65.
Hsiao JC, Chung CS, Chang W. Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. J Virol. 1999;73(10):8750–61.
Bisht H, Weisberg AS, Moss B. Vaccinia virus l1 protein is required for cell entry and membrane fusion. J Virol. 2008;82(17):8687–94.
Unger B, Traktman P. Vaccinia virus morphogenesis: A13 phosphoprotein is required for assembly of mature virions. J Virol. 2004;78(16):8885–901.
Monticelli SR, Earley AK, Stone R, Norbury CC, Ward BM. Vaccinia virus glycoproteins A33, A34, and B5 form a complex for efficient endoplasmic reticulum to trans-golgi network transport. J Virol. 2020;94(7):e02155–219.
Crickard L, Babas T, Seth S, Silvera P, Koriazova L, Crotty S. Protection of rabbits and immunodeficient mice against lethal poxvirus infections by human monoclonal antibodies. PLoS ONE. 2012;7(11):e48706.
Tamir H, Noy-Porat T, Melamed S, Cherry-Mimran L, Barlev-Gross M, Alcalay R, et al. Synergistic effect of 2 human-like monoclonal antibodies confers protection against orthopoxvirus infection. Nat Commun. 2024;15(1):3265.
Esqueda A, Sun H, Bonner J, Lai H, Jugler C, Kibler KV, et al. A monoclonal antibody produced in glycoengineered plants potently neutralizes monkeypox virus. Vaccines. 2023;11(7):1179.
Zeng J, Li Y, Jiang L, Luo L, Wang Y, Wang H, et al. Monkeypox multi-antigen mRNA vaccine candidates by a simplified manufacturing strategy afford efficient protection against lethal orthopoxvirus challenge. Emerg Microbes Infect. 2023;12(1):2204151.
Xu C, Meng X, Yan B, Crotty S, Deng J, Xiang Y. An epitope conserved in orthopoxvirus A13 envelope protein is the target of neutralizing and protective antibodies. Virology. 2011;418(1):67–73.
Li M, Ren Z, Wang Y, Jiang Y, Yang M, Li D, et al. Three neutralizing mabs induced by MPXV A29L protein recognizing different epitopes act synergistically against orthopoxvirus. Emerg Microbes Infect. 2023;12(2):2223669.
Meng N, Cheng X, Sun M, Zhang Y, Sun X, Liu X, et al. Screening, expression and identification of nanobody against monkeypox virus A35R. Int J Nanomedicine. 2023;18:7173–81.
Zhao R, Wu L, Sun J, Liu D, Han P, Gao Y, et al. Two noncompeting human neutralizing antibodies targeting MPXV B6 show protective effects against orthopoxvirus infections. Nat Commun. 2024;15(1):4660.
Moss B. Membrane fusion during poxvirus entry. Semin Cell Dev Biol. 2016;60:89–96.
Al-Kuraishy HM, Al-Gareeb AI, Hetta HF, Alexiou A, Papadakis M, Batiha GE. Monkeypox epidemic at the door: should we remain idly by or prepare strongly?. AMB Express. 2023;13(1):5.
Foo CH, Lou H, Whitbeck JC, Ponce-de-León M, Atanasiu D, Eisenberg RJ, et al. Vaccinia virus L1 binds to cell surfaces and blocks virus entry independently of glycosaminoglycans. Virology. 2009;385(2):368–82.
Carter GC, Law M, Hollinshead M, Smith GL. Entry of the vaccinia virus intracellular mature virion and its interactions with glycosaminoglycans. J Gen Virol. 2005;86(Pt 5):1279–90.
Wang Y, Yang K, Zhou H. Immunogenic proteins and potential delivery platforms for monkeypox virus vaccine development: a rapid review. Int J Biol Macromol. 2023;245:125515.
Schmidt FI, Bleck CK, Helenius A, Mercer J. Vaccinia extracellular virions enter cells by macropinocytosis and acid-activated membrane rupture. Embo J. 2011;30(17):3647–61.
Roberts KL, Breiman A, Carter GC, Ewles HA, Hollinshead M, Law M, et al. Acidic residues in the membrane-proximal stalk region of vaccinia virus protein B5 are required for glycosaminoglycan-mediated disruption of the extracellular enveloped virus outer membrane. J Gen Virol. 2009;90(Pt 7):1582–91.
Law M, Carter GC, Roberts KL, Hollinshead M, Smith GL. Ligand-induced and nonfusogenic dissolution of a viral membrane. Proc Natl Acad Sci USA. 2006;103(15):5989–94.
Lant S, Maluquer de Motes C. Poxvirus interactions with the host ubiquitin system. Pathogens. 2021;10(8):1034.
Mercer J, Snijder B, Sacher R, Burkard C, Bleck CK, Stahlberg H, et al. RNAi screening reveals proteasome- and cullin3-dependent stages in vaccinia virus infection. Cell Rep. 2012;2(4):1036–47.
Liu L, Cooper T, Howley PM, Hayball JD. From crescent to mature virion: vaccinia virus assembly and maturation. Viruses. 2014;6(10):3787–808.
Maruri-Avidal L, Weisberg AS, Moss B. Direct formation of vaccinia virus membranes from the endoplasmic reticulum in the absence of the newly characterized L2-interacting protein A30.5. J Virol. 2013;87(22):12313–26.
Tolonen N, Doglio L, Schleich S, Krijnse LJ. Vaccinia virus DNA replication occurs in endoplasmic reticulum-enclosed cytoplasmic mini-nuclei. Mol Biol Cell. 2001;12(7):2031–46.
Breiman A, Carpentier DCJ, Ewles HA, Smith GL. Transport and stability of the vaccinia virus A34 protein is affected by the A33 protein. J Gen Virol. 2013;94(Pt 4):720–5.
Perdiguero B, Blasco R. Interaction between vaccinia virus extracellular virus envelope A33 and B5 glycoproteins. J Virol. 2006;80(17):8763–77.
Abebaw D, Akelew Y, Adugna A, Teffera ZH, Tegegne BA, Fenta A, et al. Antigen recognition and immune response to monkeypox virus infection: implications for monkeypox vaccine design—a narrative review. Infez Med. 2025;33(2):151–62.
Croft NP, Smith SA, Wong YC, Tan CT, Dudek NL, Flesch IE, et al. Kinetics of antigen expression and epitope presentation during virus infection. PLoS Pathog. 2013;9(1):e1003129.
Serna A, Ramirez MC, Soukhanova A, Sigal LJ. Cutting edge: Efficient MHC class I cross-presentation during early vaccinia infection requires the transfer of proteasomal intermediates between antigen donor and presenting cells. J Immunol. 2003;171(11):5668–72.
Thiele F, Tao S, Zhang Y, Muschaweckh A, Zollmann T, Protzer U, et al. Modified vaccinia virus ankara-infected dendritic cells present CD4+ T-cell epitopes by endogenous major histocompatibility complex class II presentation pathways. J Virol. 2015;89(5):2698–709.
Lum FM, Torres-Ruesta A, Tay MZ, Lin RTP, Lye DC, Rénia L, et al. Monkeypox: disease epidemiology, host immunity and clinical interventions. Nat Rev Immunol. 2022;22(10):597–613.
Song H, Josleyn N, Janosko K, Skinner J, Reeves RK, Cohen M, et al. Monkeypox virus infection of rhesus macaques induces massive expansion of natural killer cells but suppresses natural killer cell functions. PLoS ONE. 2013;8(10):e77804.
MacLeod MK, Clambey ET, Kappler JW, Marrack P. CD4 memory T cells: What are they and what can they do?. Semin Immunol. 2009;21(2):53–61.
Edghill-Smith Y, Golding H, Manischewitz J, King LR, Scott D, Bray M, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11(7):740–7.
Hickman HD, Reynoso GV, Ngudiankama BF, Rubin EJ, Magadán JG, Cush SS, et al. Anatomically restricted synergistic antiviral activities of innate and adaptive immune cells in the skin. Cell Host Microbe. 2013;13(2):155–68.
Kennedy RB, Ovsyannikova IG, Jacobson RM, Poland GA. The immunology of smallpox vaccines. Curr Opin Immunol. 2009;21(3):314–20.
Grifoni A, Zhang Y, Tarke A, Sidney J, Rubiro P, Reina-Campos M, et al. Defining antigen targets to dissect vaccinia virus and monkeypox virus-specific T cell responses in humans. Cell Host Microbe. 2022;30(12):1662–70.e4.
Alzhanova D, Hammarlund E, Reed J, Meermeier E, Rawlings S, Ray CA, et al. T cell inactivation by poxviral B22 family proteins increases viral virulence. PLoS Pathog. 2014;10(5):e1004123.
Li H, Huang QZ, Zhang H, Liu ZX, Chen XH, Ye LL, et al. The land-scape of immune response to monkeypox virus. EBioMedicine. 2023;87:104424.
Saghazadeh A, Rezaei N. Poxviruses and the immune system: implications for monkeypox virus. Int Immunopharmacol. 2022;113(Pt A):109364.
Karem KL, Reynolds M, Hughes C, Braden Z, Nigam P, Crotty S, et al. Monkeypox-induced immunity and failure of childhood smallpox vaccination to provide complete protection. Clin Vaccine Immunol. 2007;14(10):1318–27.
Gilchuk I, Gilchuk P, Sapparapu G, Lampley R, Singh V, Kose N, et al. Cross-neutralizing and protective human antibody specificities to poxvirus infections. Cell. 2016;167(3):684–94.e9.
Brown JN, Estep RD, Lopez-Ferrer D, Brewer HM, Clauss TR, Manes NP, et al. Characterization of macaque pulmonary fluid proteome during monkeypox infection: dynamics of host response. Mol Cell Proteomics. 2010;9(12):2760–71.
Liszewski MK, Leung MK, Hauhart R, Buller RM, Bertram P, Wang X, et al. Structure and regulatory profile of the monkeypox inhibitor of complement: comparison to homologs in vaccinia and variola and evidence for dimer formation. J Immunol. 2006;176(6):3725–34.
Ghate SD, Suravajhala P, Patil P, Vangala RK, Shetty P, Rao RSP. Molecular detection of monkeypox and related viruses: challenges and opportunities. Virus Genes. 2023;59(3):343–50.
Langer J, Jimenez de Aberasturi D, Aizpurua J, Alvarez-Puebla RA, Auguié B, Baumberg JJ, et al. Present and future of surface-enhanced raman scattering. ACS Nano. 2020;14(1):28–117.
Babu PJ, Tirkey A, Paul AA, Kristollari K, Barman J, Panda K, et al. Advances in nano silver-based biomaterials and their biomedical applications. Eng Regen. 2024;5(3):326–41.
Awiaz G, Lin J, Wu A. Recent advances of Au@Ag core-shell SERS-based biosensors. Exploration. 2023;3(1):20220072.
Sun H, Miao Y, Yang X, Guo L, Li Q, Wang J, et al. Rapid identification of A29l antibodies based on mrna immunization and high-throughput single B cell sequencing to detect monkeypox virus. Emerg Microbes Infect. 2024;13(1):2332665.
Townsend MB, MacNeil A, Reynolds MG, Hughes CM, Olson VA, Damon IK, et al. Evaluation of the tetracore orthopox biothreat® antigen detection assay using laboratory-grown orthopoxviruses and rash illness clinical specimens. J Virol Methods. 2013;187(1):37–42.
Ye L, Lei X, Xu X, Xu L, Kuang H, Xu C. Gold-based paper for antigen detection of monkeypox virus. Analyst. 2023;148(5):985–94.
Yu Q, Li J, Zheng S, Xia X, Xu C, Wang C, et al. Molybdenum disulfide-loaded multilayer aunps with colorimetric-sers dual-signal enhancement activities for flexible immunochromatographic diagnosis of monkeypox virus. J Hazard Mater. 2023;459:132136.
Yan H, Su J, Tian L, Li Q, Feng X, Zhang J, et al. A rapid and sensitive fluorescent chromatography with cloud system for mpxv point-of-care diagnosis. Anal Chim Acta. 2024;1302:342514.
Wang W, Wang J, Hu Z, Yan X, Gao Q, Li X, et al. Advancing aggregation‐induced emission‐derived biomaterials in viral, tuberculosis, and fungal infectious diseases. Aggregate. 2024.
Li Y, Cui Z, Huang L, Zhang D, Shen Y, Cheng J, et al. Aggregation-based analytical chemistry in point-of-care nanosensors. Aggregate. 2024;5(5):e559.
Shu J, Li Y, Cai H, Fu Q, Li C, Yuan J, et al. Ultrabright nir aiegen nanoparticles-enhanced lateral flow immunoassay platform for accurate diagnostics of complex samples. Aggregate. 2024;5(4):e551.
Yang X, Cheng X, Wei H, Tu Z, Rong Z, Wang C, et al. Fluorescence-enhanced dual signal lateral flow immunoassay for flexible and ultrasensitive detection of monkeypox virus. J Nanobiotechnol. 2023;21(1):450.
Chandran M, Chellasamy G, Veerapandian M, Dhanasekaran B, Kumar Arumugasamy S, Govindaraju S, et al. Fabrication of label-free immunoprobe for monkeypox A29 detection using one-step electrodeposited molybdenum oxide-graphene quantum rods. J Colloid Interface Sci. 2024;660:412–22.
de Lima LF, Barbosa PP, Simeoni CL, de Paula RFO, Proenca-Modena JL, de Araujo WR. Electrochemical paper-based nanobiosensor for rapid and sensitive detection of monkeypox virus. ACS Appl Mater Interfaces. 2023;15(50):58079–91.
Fan Z, Liu Y, Ye Y, Liao Y. Functional probes for the diagnosis and treatment of infectious diseases. Aggregate. 2024;5(6):e620.
Han C, Liu Q, Luo X, Zhao J, Zhang Z, He J, et al. Development of a CRISPR/Cas12a-mediated aptasensor for mpox virus antigen detection. Biosens Bioelectron. 2024;257:116313.
Cai S, Ren R, He J, Wang X, Zhang Z, Luo Z, et al. Selective single-molecule nanopore detection of monkeypox A29 protein directly in biofluids. Nano Lett. 2023;23(24):11438–46.
Organization WH, Target product profiles for tests used for monkeypox (monkeypox) diagnosis. World Health Organization: 2023.
Trivedi J, Yasir M, Maurya RK, Tripathi AS. Aptamer-based theranostics in oncology: design strategies and limitations. BIO Integr. 2024;5(1):1–20.
Li T, Qian C, Gu Y, Zhang J, Li S, Xia N. Current progress in the development of prophylactic and therapeutic vaccines. Sci China Life Sci. 2023;66(4):679–710.
Poria R, Kala D, Nagraik R, Dhir Y, Dhir S, Singh B, et al. Vaccine development: current trends and technologies. Life Sci. 2024;336:122331.
Bartlett BL, Pellicane AJ, Tyring SK. Vaccine immunology. Dermatol Ther. 2009;22(2):104–9.
Delany I, Rappuoli R, De Gregorio E. Vaccines for the 21st century. EMBO Mol Med. 2014;6(6):708–20.
Poland GA, Kennedy RB, Tosh PK. Prevention of monkeypox with vaccines: a rapid review. Lancet Infect Dis. 2022;22(12):e349–58.
Lozano JM, Muller S. Monkeypox: potential vaccine development strategies. Trends Pharmacol Sci. 2023;44(1):15–9.
Garcia-Atutxa I, Mondragon-Teran P, Huerta-Saquero A, Villanueva-Flores F. Advancements in monkeypox vaccines development: a critical review of emerging technologies. Front Immunol. 2024;15:1456060.
Rothenburg S, Yang Z, Beard P, Sawyer SL, Titanji B, Gonsalves G, et al. Monkeypox emergency: urgent questions and perspectives. Cell. 2022;185(18):3279–81.
Schmidt FI, Bleck CK, Mercer J. Poxvirus host cell entry. Curr Opin Virol. 2012;2(1):20–7.
Van Vliet K, Mohamed MR, Zhang L, Villa NY, Werden SJ, Liu J, et al. Poxvirus proteomics and virus-host protein interactions. Microbiol Mol Biol Rev. 2009;73(4):730–49.
Weaver JR, Isaacs SN. Monkeypox virus and insights into its immunomodulatory proteins. Immunol Rev. 2008;225:96–113.
Papukashvili D, Rcheulishvili N, Liu C, Wang X, He Y, Wang PG. Strategy of developing nucleic acid-based universal monkeypox vaccine candidates. Front Immunol. 2022;13:1050309.
Keasey S, Pugh C, Tikhonov A, Chen G, Schweitzer B, Nalca A, et al. Proteomic basis of the antibody response to monkeypox virus infection examined in cynomolgus macaques and a comparison to human smallpox vaccination. PLoS ONE. 2010;5(12):e15547.
Moss B. Poxvirus cell entry: How many proteins does it take?. Viruses. 2012;4(5):688–707.
Galmiche MC, Goenaga J, Wittek R, Rindisbacher L. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology. 1999;254(1):71–80.
Lai CF, Gong SC, Esteban M. The purified 14-kilodalton envelope protein of vaccinia virus produced in escherichia coli induces virus immunity in animals. J Virol. 1991;65(10):5631–5.
Lustig S, Fogg C, Whitbeck JC, Eisenberg RJ, Cohen GH, Moss B. Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. J Virol. 2005;79(21):13454–62.
Hooper JW, Custer DM, Thompson E. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology. 2003;306(1):181–95.
Zhang Y, Zhou Y, Pei R, Chen X, Wang Y. Potential threat of human pathogenic orthopoxviruses to public health and control strategies. J Biosaf Biosecur. 2023;5(1):1–7.
Hooper JW, Custer DM, Schmaljohn CS, Schmaljohn AL. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology. 2000;266(2):329–39.
Fogg C, Lustig S, Whitbeck JC, Eisenberg RJ, Cohen GH, Moss B. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J Virol. 2004;78(19):10230–7.
Hooper JW, Thompson E, Wilhelmsen C, Zimmerman M, Ichou MA, Steffen SE, et al. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J Virol. 2004;78(9):4433–43.
Hooper JW, Golden JW, Ferro AM, King AD. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007;25(10):1814–23.
Hirao LA, Draghia-Akli R, Prigge JT, Yang M, Satishchandran A, Wu L, et al. Multivalent smallpox DNA vaccine delivered by intradermal electroporation drives protective immunity in nonhuman primates against lethal monkeypox challenge. J Infect Dis. 2011;203(1):95–102.
Brown E, Senkevich TG, Moss B. Vaccinia virus F9 virion membrane protein is required for entry but not virus assembly, in contrast to the related L1 protein. J Virol. 2006;80(19):9455–64.
Davies DH, McCausland MM, Valdez C, Huynh D, Hernandez JE, Mu Y, et al. Vaccinia virus h3l envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J Virol. 2005;79(18):11724–33.
Freyn AW, Atyeo C, Earl PL, Americo JL, Chuang GY, Natarajan H, et al. An mpox virus mRNA-lipid nanoparticle vaccine confers protection against lethal orthopoxviral challenge. Sci Transl Med. 2023;15(716):eadg3540.
Mucker EM, Freyn AW, Bixler SL, Cizmeci D, Atyeo C, Earl PL, et al. Comparison of protection against monkeypox following mRNA or modified vaccinia ankara vaccination in nonhuman primates. Cell. 2024;187(20):5540–53.e10.
Zhang RR, Wang ZJ, Zhu YL, Tang W, Zhou C, Zhao SQ, et al. Rational development of multicomponent mRNA vaccine candidates against monkeypox. Emerg Microbes Infect. 2023;12(1):2192815.
Zhang N, Cheng X, Zhu Y, Mo O, Yu H, Zhu L, et al. Multivalent mRNA vaccines against monkeypox enveloped or mature viron surface antigens demonstrate robust immune response and neutralizing activity. Sci China Life Sci. 2023;66(10):2329–41.
Hou F, Zhang Y, Liu X, Murad YM, Xu J, Yu Z, et al. mRNA vaccines encoding fusion proteins of monkeypox virus antigens protect mice from vaccinia virus challenge. Nat Commun. 2023;14(1):5925.
Fang Z, Monteiro VS, Renauer PA, Shang X, Suzuki K, Ling X, et al. Polyvalent mrna vaccination elicited potent immune response to monkeypox virus surface antigens. Cell Res. 2023;33(5):407–10.
Ye T, Zhou J, Guo C, Zhang K, Wang Y, Liu Y, et al. Polyvalent monkeypox mRNA vaccines elicit robust immune responses and confer potent protection against vaccinia virus. Cell Rep. 2024;43(6):114269.
Yang X, Hu C, Yang X, Yang X, Hu X, Wang X, et al. Evaluation and comparison of immune responses induced by 2 monkeypox mRNA vaccine candidates in mice. J Med Virol. 2023;95(10):e29140.
Su C, Li S, Wen Y, Geng X, Yin Q, Wang Y, et al. A quadrivalent mRNA immunization elicits potent immune responses against multiple orthopoxviral antigens and neutralization of monkeypox virus in rodent models. Vaccines. 2024;12(4):385.
Hendrickson RC, Wang C, Hatcher EL, Lefkowitz EJ. Orthopoxvirus genome evolution: the role of gene loss. Viruses. 2010;2(9):1933–67.
Fenner F, Wittek R, Dumbell K. The pathogenesis, pathology, and immunology of orthopoxvirus infections. The Orthopoxviruses. 1989:85–141.
Cheng F, Wang Y, Bai Y, Liang Z, Mao Q, Liu D, et al. Research advances on the stability of mRNA vaccines. Viruses. 2023;15(3):668.
Guasp P, Reiche C, Sethna Z, Balachandran VP. RNA vaccines for cancer: principles to practice. Cancer Cell. 2024;42(7):1163–84.
Greenberg RN, Overton ET, Haas DW, Frank I, Goldman M, von Krempelhuber A, et al. Safety, immunogenicity, and surrogate markers of clinical efficacy for modified vaccinia ankara as a smallpox vaccine in HIV-infected subjects. J Infect Dis. 2013;207(5):749–58.
Wang X, Gu Z, Sheng S, Song R, Jin R. The current state and progress of monkeypox vaccine research. China CDC Wkly. 2024;6(7):118–25.
Fogg CN, Americo JL, Lustig S, Huggins JW, Smith SK, Damon I, et al. Adjuvant-enhanced antibody responses to recombinant proteins correlate with protection of mice and monkeys to orthopoxvirus challenges. Vaccine. 2007;25(15):2787–99.
Buchman GW, Cohen ME, Xiao Y, Richardson-Harman N, Silvera P, DeTolla LJ, et al. A protein-based smallpox vaccine protects non-human primates from a lethal monkeypox virus challenge. Vaccine. 2010;28(40):6627–36.
Benhnia MR, McCausland MM, Moyron J, Laudenslager J, Granger S, Rickert S, et al. Vaccinia virus extracellular enveloped virion neutralization in vitro and protection in vivo depend on complement. J Virol. 2009;83(3):1201–15.
Benhnia MR, McCausland MM, Laudenslager J, Granger SW, Rickert S, Koriazova L, et al. Heavily isotype-dependent protective activities of human antibodies against vaccinia virus extracellular virion antigen B5. J Virol. 2009;83(23):12355–67.
Tang D, Liu X, Lu J, Fan H, Xu X, Sun K, et al. Recombinant proteins A29L, M1R, A35R, and B6R vaccination protects mice from monkeypox virus challenge. Front Immunol. 2023;14:1203410.
Yang X, Yang X, Du S, Hu C, Yang X, Wang X, et al. A subunit vaccine candidate composed of monkeypox virus A29L, M1R, A35R, and B6R elicits robust immune response in mice. Vaccines. 2023;11(9):1420.
Lin W, Shen C, Li M, Ma S, Liu C, Huang J, et al. Programmable macrophage vesicle based bionic self-adjuvanting vaccine for immunization against monkeypox virus. Adv Sci. 2025;12(1):e2408608.
Wang H, Yin P, Zheng T, Qin L, Li S, Han P, et al. Rational design of a ‘two-in-one’ immunogen dam drives potent immune response against monkeypox virus. Nat Immunol. 2024;25(2):307–15.
Zhang L. Multi-epitope vaccines: a promising strategy against tumors and viral infections. Cell Mol Immunol. 2018;15(2):182–4.
Tiwary P, Oswal K, Varghese R, Anchan H, Oswal M. Multi-epitope vaccines: charting a new frontier in monkeypox prevention and control. Hum Cell. 2025;38(5):126.
Oli AN, Obialor WO, Ifeanyichukwu MO, Odimegwu DC, Okoyeh JN, Emechebe GO, et al. Immunoinformatics and vaccine development: an overview. Immunotargets Ther. 2020;9:13–30.
Bidmos FA, Siris S, Gladstone CA, Langford PR. Bacterial vaccine antigen discovery in the reverse vaccinology 2.0 era: progress and challenges. Front Immunol. 2018;9:2315.
Tan C, Zhu F, Pan P, Wu A, Li C. Development of multi-epitope vaccines against the monkeypox virus based on envelope proteins using immunoinformatics approaches. Front Immunol. 2023;14:1112816.
Roberts KL, Smith GL. Vaccinia virus morphogenesis and dissemination. Trends Microbiol. 2008;16(10):472–9.
Suleman M, Rashid F, Ali S, Sher H, Luo S, Xie L, et al. Immunoinformatic-based design of immune-boosting multiepitope subunit vaccines against monkeypox virus and validation through molecular dynamics and immune simulation. Front Immunol. 2022;13:1042997.
Yousaf M, Ismail S, Ullah A, Bibi S. Immuno-informatics profiling of monkeypox virus cell surface binding protein for designing a next-generation multi-valent peptide-based vaccine. Front Immunol. 2022;13:1035924.
Shantier SW, Mustafa MI, Abdelmoneim AH, Fadl HA, Elbager SG, Makhawi AM. Novel multi epitope-based vaccine against monkeypox virus: vaccinomic approach. Sci Rep. 2022;12(1):15983.
Sanami S, Nazarian S, Ahmad S, Raeisi E, Tahir Ul Qamar M, Tahmasebian S, et al. In silico design and immunoinformatics analysis of a universal multi-epitope vaccine against monkeypox virus. PLoS ONE. 2023;18(5):e0286224.
de Araújo LP, de Melo Santos NC, Corsetti PP, de Almeida LA. Immunoinformatic approach for rational identification of immunogenic peptides against host entry and/or exit monkeypox proteins and potential multiepitope vaccine construction. J Infect Dis. 2024;229(Supplement_2):S285–92.
Sette A, Rappuoli R. Reverse vaccinology: developing vaccines in the era of genomics. Immunity. 2010;33(4):530–41.
Jin Y, Fayyaz A, Liaqat A, Khan A, Alshammari A, Wang Y, et al. Proteomics-based vaccine targets annotation and design of subunit and mRNA-based vaccines for monkeypox virus (mpxv) against the recent outbreak. Comput Biol Med. 2023;159:106893.
Singh S, Rao A, Kumar K, Mishra A, Prajapati VK. Translational vaccinomics and structural filtration algorithm to device multiepitope vaccine for catastrophic monkeypox virus. Comput Biol Med. 2023;153:106497.
Waqas M, Aziz S, Liò P, Khan Y, Ali A, Iqbal A, et al. Immunoinformatics design of multivalent epitope vaccine against monkeypox virus and its variants using membrane-bound, enveloped, and extracellular proteins as targets. Front Immunol. 2023;14:1091941.
Aiman S, Alhamhoom Y, Ali F, Rahman N, Rastrelli L, Khan A, et al. Multi-epitope chimeric vaccine design against emerging monkeypox virus via reverse vaccinology techniques-a bioinformatics and immunoinformatics approach. Front Immunol. 2022;13:985450.
Akhtar N, Kaushik V, Grewal RK, Wani AK, Suwattanasophon C, Choowongkomon K, et al. Immunoinformatics-aided design of a peptide based multiepitope vaccine targeting glycoproteins and membrane proteins against monkeypox virus. Viruses. 2022;14(11):2374.
Hammarlund E, Dasgupta A, Pinilla C, Norori P, Früh K, Slifka MK. Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proc Natl Acad Sci USA. 2008;105(38):14567–72.
Pritam M. Exploring the whole proteome of monkeypox virus to design B-cell epitope-based oral vaccines using immunoinformatics approaches. Int J Biol Macromol. 2023;252:126498.
Swetha RG, Basu S, Ramaiah S, Anbarasu A. Multi-epitope vaccine for monkeypox using pan-genome and reverse vaccinology approaches. Viruses. 2022;14(11):2504.
Alsaiari AA, Hakami MA, Alotaibi BS, Alkhalil SS, Alkhorayef N, Khan K, et al. Delineating multi-epitope vaccine designing from membrane protein CL5 against all monkeypox strains: a pangenome reverse vaccinology approach. J Biomol Struct Dyn. 2024;42(16):8385–406.
Bloch EM, Sullivan DJ, Shoham S, Tobian AAR, Casadevall A, Gebo KA. The potential role of passive antibody-based therapies as treatments for monkeypox. MBio. 2022;13(6):e0286222.
Thet AK, Kelly PJ, Kasule SN, Shah AK, Chawala A, Latif A, et al. The use of vaccinia immune globulin in the treatment of severe monkeypox. Virus infection in human immunodeficiency virus/aids. Clin Infect Dis. 2023;76(9):1671–3.
Gu X, Zhang Y, Jiang W, Wang D, Lu J, Gu G, et al. Protective human anti-poxvirus monoclonal antibodies are generated from rare memory B cells isolated by multicolor antigen tetramers. Vaccines. 2022;10(7):1084.
Wittek R. Vaccinia immune globulin: current policies, preparedness, and product safety and efficacy. Int J Infect Dis. 2006;10(3):193–201.
Xiao Y, Isaacs SN. Therapeutic vaccines and antibodies for treatment of orthopoxvirus infections. Viruses. 2010;2(10):2381–403.
Bell E, Shamim M, Whitbeck JC, Sfyroera G, Lambris JD, Isaacs SN. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology. 2004;325(2):425–31.
Shchelkunov SN, Totmenin AV, Babkin IV, Safronov PF, Ryazankina OI, Petrov NA, et al. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett. 2001;509(1):66–70.
Guarner J, Del Rio C, Malani PN. Monkeypox in 2022-what clinicians need to know. JAMA. 2022;328(2):139–40.
Piparva KG, Fichadiya N, Joshi T, Malek S. Monkeypox: from emerging trends to therapeutic concerns. Cureus. 2024;16(4):e58866.
Khalil A, Samara A, O’Brien P, Ladhani SN. Treatment and prevention of monkeypox in pregnant people and young children. Lancet Infect Dis. 2023;23(4):396–7.
Khalil A, Samara A, O’Brien P, Coutinho CM, Duarte G, Quintana SM, et al. Monkeypox in pregnancy: update on current outbreak. Lancet Infect Dis. 2022;22(11):1534–5.
Parker S, D’Angelo J, Buller RM, Smee DF, Lantto J, Nielsen H, et al. A human recombinant analogue to plasma-derived vaccinia immunoglobulin prophylactically and therapeutically protects against lethal orthopoxvirus challenge. Antivir Res. 2021;195:105179.
Mitchell LS, Colwell LJ. Analysis of nanobody paratopes reveals greater diversity than classical antibodies. Protein Eng Des Sel. 2018;31(7–8):267–75.
Liu W, Song H, Chen Q, Yu J, Xian M, Nian R, et al. Recent advances in the selection and identification of antigen-specific nanobodies. Mol Immunol. 2018;96:37–47.
Allegra A, Innao V, Gerace D, Vaddinelli D, Allegra AG, Musolino C. Nanobodies and cancer: current status and new perspectives. Cancer Invest. 2018;36(4):221–37.
Yu H, Mao G, Pei Z, Cen J, Meng W, Wang Y, et al. In vitro affinity maturation of nanobodies against monkeypox virus A29 protein based on computer-aided design. Molecules. 2023;28(19):6838.
Wolffe EJ, Vijaya S, Moss B. A myristylated membrane protein encoded by the vaccinia virus l1r open reading frame is the target of potent neutralizing monoclonal antibodies. Virology. 1995;211(1):53–63.
McNulty MJ, Nandi S, McDonald KA. Technoeconomic modeling and simulation for plant-based manufacturing of recombinant proteins. Methods Mol Biol. 2022;2480:159–89.
Nandi S, Kwong AT, Holtz BR, Erwin RL, Marcel S, McDonald KA. Techno-economic analysis of a transient plant-based platform for monoclonal antibody production. MAbs. 2016;8(8):1456–66.
Chen Q. Glycoengineering of plants yields glycoproteins with polysialylation and other defined N-glycoforms. Proc Natl Acad Sci USA. 2016;113(34):9404–6.
Pereira NA, Chan KF, Lin PC, Song Z. The, “less-is-more” in therapeutic antibodies: afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. MAbs. 2018;10(5):693–711.
Marusic C, Pioli C, Stelter S, Novelli F, Lonoce C, Morrocchi E, et al. N-glycan engineering of a plant-produced anti-CD20-HIL-2 immunocytokine significantly enhances its effector functions. Biotechnol Bioeng. 2018;115(3):565–76.
Matho MH, Schlossman A, Meng X, Benhnia MR, Kaever T, Buller M, et al. Structural and functional characterization of anti-A33 antibodies reveal a potent cross-species orthopoxviruses neutralizer. PLoS Pathog. 2015;11(9):e1005148.
Chen Z, Earl P, Americo J, Damon I, Smith SK, Yu F, et al. Characterization of chimpanzee/human monoclonal antibodies to vaccinia virus A33 glycoprotein and its variola virus homolog in vitro and in a vaccinia virus mouse protection model. J Virol. 2007;81(17):8989–95.
Chen Z, Earl P, Americo J, Damon I, Smith SK, Zhou YH, et al. Chimpanzee/human mAbs to vaccinia virus B5 protein neutralize vaccinia and smallpox viruses and protect mice against vaccinia virus. Proc Natl Acad Sci U S A. 2006;103(6):1882–7.
Saadh MJ, Ghadimkhani T, Soltani N, Abbassioun A, Daniel Cosme Pecho R, Taha A, et al. Progress and prospects on vaccine development against monkeypox infection. Microb Pathog. 2023;180:106156.
Mucker EM, Wollen-Roberts SE, Kimmel A, Shamblin J, Sampey D, Hooper JW. Intranasal monkeypox marmoset model: prophylactic antibody treatment provides benefit against severe monkeypox virus disease. PLoS Negl Trop Dis. 2018;12(6):e0006581.
Mucker EM, Thiele-Suess C, Baumhof P, Hooper JW. Lipid nanoparticle delivery of unmodified mRNAs encoding multiple monoclonal antibodies targeting poxviruses in rabbits. Mol Ther Nucleic Acids. 2022;28:847–58.
Chi H, Zhao SQ, Chen RY, Suo XX, Zhang RR, Yang WH, et al. Rapid development of double-hit mRNA antibody cocktail against orthopoxviruses. Signal Transduct Target Ther. 2024;9(1):69.
Wang W, Mo W, Xiao X, Cai M, Feng S, Wang Y, et al. Antibiotic-loaded lactoferrin nanoparticles as a platform for enhanced infection therapy through targeted elimination of intracellular bacteria. Asian J Pharm Sci. 2024;19(4):100926.
Zhang L, Huang J, Buratto D, Han P, Yang Z, Zhou R. A pH-responsive nanoparticle delivery system containing dihydralazine and doxorubicin-based prodrug for enhancing antitumor efficacy. Aggregate. 2023;5(1):e434.
Wang W, Guo H, Lin S, Xiao X, Liu Y, Wang Y, et al. Biosafety materials for tuberculosis treatment. Biosaf Health. 2022;4(4):258–68.
Hagedorn L, Jürgens DC, Merkel OM, Winkeljann B. Endosomal escape mechanisms of extracellular vesicle-based drug carriers: lessons for lipid nanoparticle design. Extracell Vesicles Circ Nucl Acids. 2024;5(3):344–57.
Liang F, An X, Wang R, Wu W, Yang L, Zheng Y, et al. Microalgae-based drug delivery system for tumor microenvironment photo-modulating and synergistic chemo-photodynamic therapy of osteosarcoma. Eng Regen. 2024;5(2):199–209.
Huang H, Lang Y, Wang S, Zhou M. Microalgae-based drug delivery systems in biomedical applications. Eng Regen. 2024;5(3):361–74.
Wu Y, Liu Y, Wang T, Jiang Q, Xu F, Liu Z. Living cell for drug delivery. Eng Regen. 2022;3(2):131–48.
Ijaz M, Hasan I, Chaudhry TH, Huang R, Zhang L, Hu Z, et al. Bacterial derivatives mediated drug delivery in cancer therapy: a new generation strategy. J Nanobiotechnol. 2024;22(1):510.
He S, Fu Y, Tan Z, Jiang Q, Huang K, Saw PE, et al. Optimization of ultra-small nanoparticles for enhanced drug delivery. BIO Integration. 2023;4(2):62–9.
Wang F, Li Z. Engineered extracellular vesicles as “supply vehicles” to alleviate type 1 diabetes. Extracell Vesicles Circ Nucl Acids. 2024;5(4):618–21.
Liu H, Song P, Zhang H, Zhou F, Ji N, Wang M, et al. Synthetic biology-based bacterial extracellular vesicles displaying BMP-2 and CXCR-4 to ameliorate osteoporosis. J Extracell Vesicles. 2024;13(4):e12429.
Liu H, Zhang Q, Wang S, Weng W, Jing Y, Su J. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: advances and perspectives. Bioact Mater. 2022;14:169–81.
Pantaleo G, Correia B, Fenwick C, Joo VS, Perez L. Antibodies to combat viral infections: development strategies and progress. Nat Rev Drug Discov. 2022;21(9):676–96.
Garegnani L, Styrmisdóttir L, Roson Rodriguez P, Escobar Liquitay CM, Esteban I, Franco JV. Palivizumab for preventing severe respiratory syncytial virus (RSV) infection in children. Cochrane Database Syst Rev. 2021;11(11):Cd013757.
Li B, Wang W, Zhao L, Li M, Yan D, Li X, et al. Aggregation-induced emission-based macrophage-like nanoparticles for targeted photothermal therapy and virus transmission blockage in monkeypox. Adv Mater. 2024;36(9):e2305378.
Wang C, Lv S, Sun Z, Xiao M, Fu H, Tian L, et al. A self-regulated phototheranostic nanosystem with single wavelength-triggered energy switching and oxygen supply for multimodal synergistic therapy of bacterial biofilm infections. Aggregate. 2024;5(5):e587.
Li X, Wang W, Gao Q, Lai S, Liu Y, Zhou S, et al. Intelligent bacteria-targeting ZIF-8 composite for fluorescence imaging-guided photodynamic therapy of drug-resistant superbug infections and burn wound healing. Exploration. 2024;4(6):20230113.
Li B, Wang W, Zhao L, Wu Y, Li X, Yan D, et al. Photothermal therapy of tuberculosis using targeting pre-activated macrophage membrane-coated nanoparticles. Nat Nanotechnol. 2024;19(6):834–45.
Chen L, Wang X, Yuan Y, Hu R, Chen Q, Zhu L, et al. Photosensitizers with aggregation-induced emission and their biomedical applications. Eng Regen. 2022;3(1):59–72.
Li B, Wang W, Song W, Zhao Z, Tan Q, Zhao Z, et al. Antiviral and anti-inflammatory treatment with multifunctional alveolar macrophage-like nanoparticles in a surrogate mouse model of COVID-19. Adv Sci. 2021;8(13):2003556.
Hu Z, Wang W, Lin Y, Guo H, Chen Y, Wang J, et al. Extracellular vesicle-inspired therapeutic strategies for the COVID-19. Adv Healthc Mater. 2024;13(29):e2402103.
Shi R, Zhan A, Li X, Kong B, Liang G. Biomimetic extracellular vesicles for the tumor targeted treatment. Eng Regen. 2023;4(4):427–37.
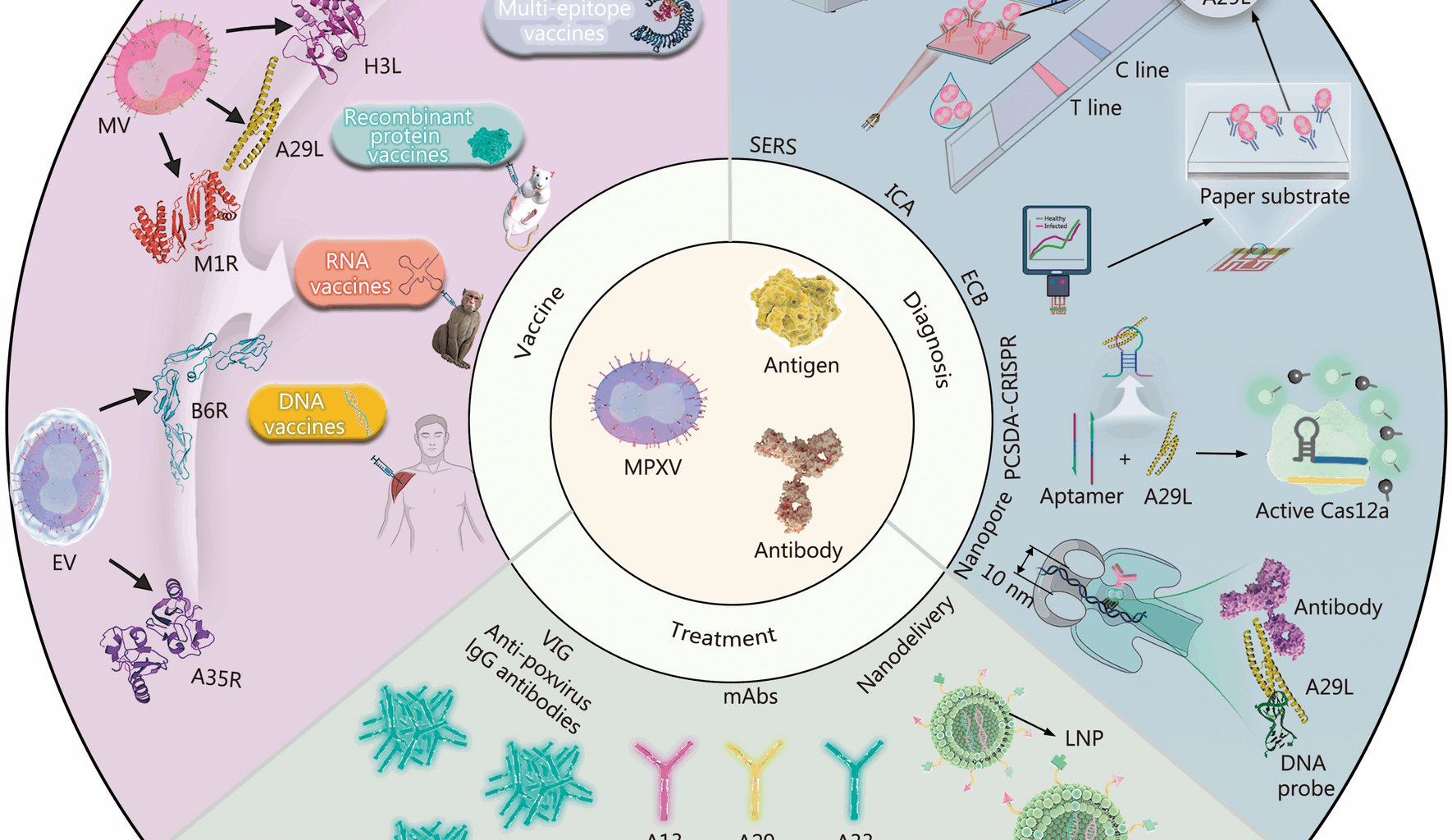
Jiang L, Xu A, Guan L, Tang Y, Chai G, Feng J, et al. A review of monkeypox: biological characteristics, epidemiology, clinical features, diagnosis, treatment, and prevention strategies. Exploration. 2024;5(2):20230112.
Ma T, Liu D, Lyu K, Gao T, Shi D, Zhao L, et al. Establishment and application of national reference panels for SARS-CoV-2 antigen detection kit. Biosaf Health. 2023;5(6):326–30.
Zhou H, Wang C, Rao J, Chen L, Ma T, Liu D, et al. The impact of sample processing on the rapid antigen detection test for SARS-CoV-2: virus inactivation, VTM selection, and sample preservation. Biosaf Health. 2021;3(5):238–43.
Forgham H, Kakinen A, Qiao R, Davis TP. Keeping up with the COVID’s—Could siRNA-based antivirals be a part of the answer? Exploration. 2022;2(6):20220012.
Kubiatowicz LJ, Mohapatra A, Krishnan N, Fang RH, Zhang L. mRNA nanomedicine: design and recent applications. Exploration. 2022;2(6):20210217.
Wang Z, Cui K, Costabel U, Zhang X. Nanotechnology-facilitated vaccine development during the coronavirus disease 2019 (COVID-19) pandemic. Exploration. 2022;2(5):20210082.
Huang Y, Mu L, Wang W. Monkeypox: epidemiology, pathogenesis, treatment and prevention. Signal Transduct Target Ther. 2022;7(1):373.
Taub DD, Ershler WB, Janowski M, Artz A, Key ML, McKelvey J, et al. Immunity from smallpox vaccine persists for decades: a longitudinal study. Am J Med. 2008;121(12):1058–64.
Viner KM, Isaacs SN. Activity of vaccinia virus-neutralizing antibody in the sera of smallpox vaccinees. Microbes Infect. 2005;7(4):579–83.
Bankuru SV, Kossol S, Hou W, Mahmoudi P, Rychtář J, Taylor D. A game-theoretic model of monkeypox to assess vaccination strategies. PeerJ. 2020;8:e9272.
Pagant S, Liberatore RA. In vivo electroporation of plasmid DNA: a promising strategy for rapid, inexpensive, and flexible delivery of anti-viral monoclonal antibodies. Pharmaceutics. 2021;13(11):1882.
Babalola BA, Akinsuyi OS, Folajimi EO, Olujimi F, Otunba AA, Chikere B, et al. Exploring the future of SARS-CoV-2 treatment after the first 2 years of the pandemic: a comparative study of alternative therapeutics. Biomed Pharmacother. 2023;165:115099.
Sun H, Chen Q, Lai H. Development of antibody therapeutics against flaviviruses. Int J Mol Sci. 2017;19(1):54.
de Almeida Oliveira A, Praia Borges Freire D, Rodrigues de Andrade A, de Miranda Marques A, da Silva Madeira L, Moreno Senna JP, et al. The landscape of neutralizing monoclonal antibodies (nAbs) for treatment and prevention of COVID-19. J Pharm Innov. 2023:1–19.
Nezvalova-Henriksen K, Langebrake C, Bauters T, Moreno-Martinez ME, Ahnfelt E, Ekelund H, et al. Implementation and operational management of marketed chimeric antigen receptor T cell (CAR-T cell) therapy-a guidance by the GOCART Coalition pharmacist working group. Bone Marrow Transplant. 2023;58(10):1069–74.
Faulkner L, Verna M, Rovelli A, Agarwal RK, Dhanya R, Parmar L, et al. Setting up and sustaining blood and marrow transplant services for children in middle-income economies: an experience-driven position paper on behalf of the EBMT. PDWP Bone Marrow Transplant. 2021;56(3):536–43.
Barocas JA, Strathdee SA. Beyond sex: human monkeypox virus is an emerging threat to marginalized populations. Open Forum Infect Dis. 2022;9(11):ofac551.
Suárez Rodríguez B, Guzmán Herrador BR, Díaz Franco A, Sánchez-Seco Fariñas MP, Del Amo VJ, Aginagalde Llorente AH, et al. Epidemiologic features and control measures during monkeypox outbreak, Spain, June 2022. Emerg Infect Dis. 2022;28(9):1847–51.
Potet J, Beran D, Ray N, Alcoba G, Habib AG, Iliyasu G, et al. Access to antivenoms in the developing world: a multidisciplinary analysis. Toxicon X. 2021;12:100086.
Johri N, Kumar D, Nagar P, Maurya A, Vengat M, Jain P. Clinical manifestations of human monkeypox infection and implications for outbreak strategy. Health Sci Rev. 2022;5:100055.
Crosato V, Formenti B, Gulletta M, Odolini S, Compostella S, Tomasoni LR, et al. Perception and awareness about monkeypox and vaccination acceptance in an at-risk population in Brescia, Italy: an investigative survey. AIDS Behav. 2024;28(5):1594–600.
Lulli LG, Baldassarre A, Mucci N, Arcangeli G. Prevention, risk exposure, and knowledge of monkeypox in occupational settings: a scoping review. Trop Med Infect Dis. 2022;7(10):276.
Zardi EM, Chello C. Human monkeypox global public health emergency. Int J Environ Res Public Health. 2022;19(24):16781.
Hirani R, Rashid D, Lewis J, Hosein-Woodley R, Issani A. Monkeypox outbreak in the age of COVID-19: a new global health emergency. Mil Med Res. 2022;9(1):55.
Wallau GL, Maciel-de-Freitas R, Schmidt-Chanasit J. An unfolding monkeypox outbreak in Europe and beyond. Mil Med Res. 2022;9(1):31.
Sun N, Meng X, Liu Y, Song D, Jiang C, Cai J. Applications of brain organoids in neurodevelopment and neurological diseases. J Biomed Sci. 2021;28(1):30.