A 59-year-old man with no relevant medical history presented to the Emergency Department at night, coming straight from the airport, reporting asthenia and jaundice. The patient disclosed a recent travel to Angola, where he had received treatment for malaria and reported engaging in unprotected sexual activity during his stay. Upon further questioning, the patient revealed that he had been hospitalized for six-days with a malaria infection and treated with intravenous artesunate. On discharge, a three-day course of oral artemether-lumefantrine was prescribed. At follow-up (third and sixth days after discharge), he showed worsening anaemia (Hb 74 and 59 g/L, respectively). Concerned about his deteriorating condition, the patient decided to leave Angola and travelled directly to Portugal, presenting to the Emergency Department upon arrival.
On initial assessment, his vital signs included a heart rate of 127 bpm, blood pressure of 120/52 mmHg, oxygen saturation of 99% on room air, and normal respiratory rate. He was afebrile. Cardiac and pulmonary examinations were unremarkable. Abdominal evaluation revealed tenderness in the right upper quadrant and hepatomegaly, with the liver palpable 4–5 cm below the costal margin. Pitting oedema was noted in both lower limbs.
Laboratory tests—including blood gas analysis, malaria testing and acute viral hepatitis panel—along with a chest x-ray and an abdominal ultrasound were promptly ordered. The blood gas analysis showed slight respiratory alkalosis (pH 7.48, pCO2 28.4 mmHg) and profound anaemia (haemoglobin [Hb] 48 g/L). The abdominal ultrasound detected hepatosplenomegaly. The chest x-ray revealed no abnormalities.
The complete blood count confirmed the severe anaemia (Hb 44 g/dL), revealed a high reticulocyte count (21% or 279 × 109/L) and normal thrombocyte count (365 × 109/L). The chemistry panel showed slightly elevated aspartate aminotransferase (43 U/L) and bilirubin levels (38.65 μmol/L total and 11.29 μmol/L direct bilirubin), markedly elevated lactate dehydrogenase (1332 U/L) and decreased haptoglobin (
Malaria testing included an Abbott BinaxNOW immunochromatographic rapid diagnostic test (RDT) and evaluation of the peripheral blood smear. The RDT came back positive for Plasmodium falciparum infection (PfHRP2 positive). The peripheral blood smear was prepared on the Sysmex SP-50, digital imaging was obtained by the Sysmex DI-60 system. The slide was evaluated using light microscopy (Nikon Eclypse E400) by the on duty clinical pathologist. The peripheral smear showed anisocytosis and polychromasia. No malaria parasites were observed.
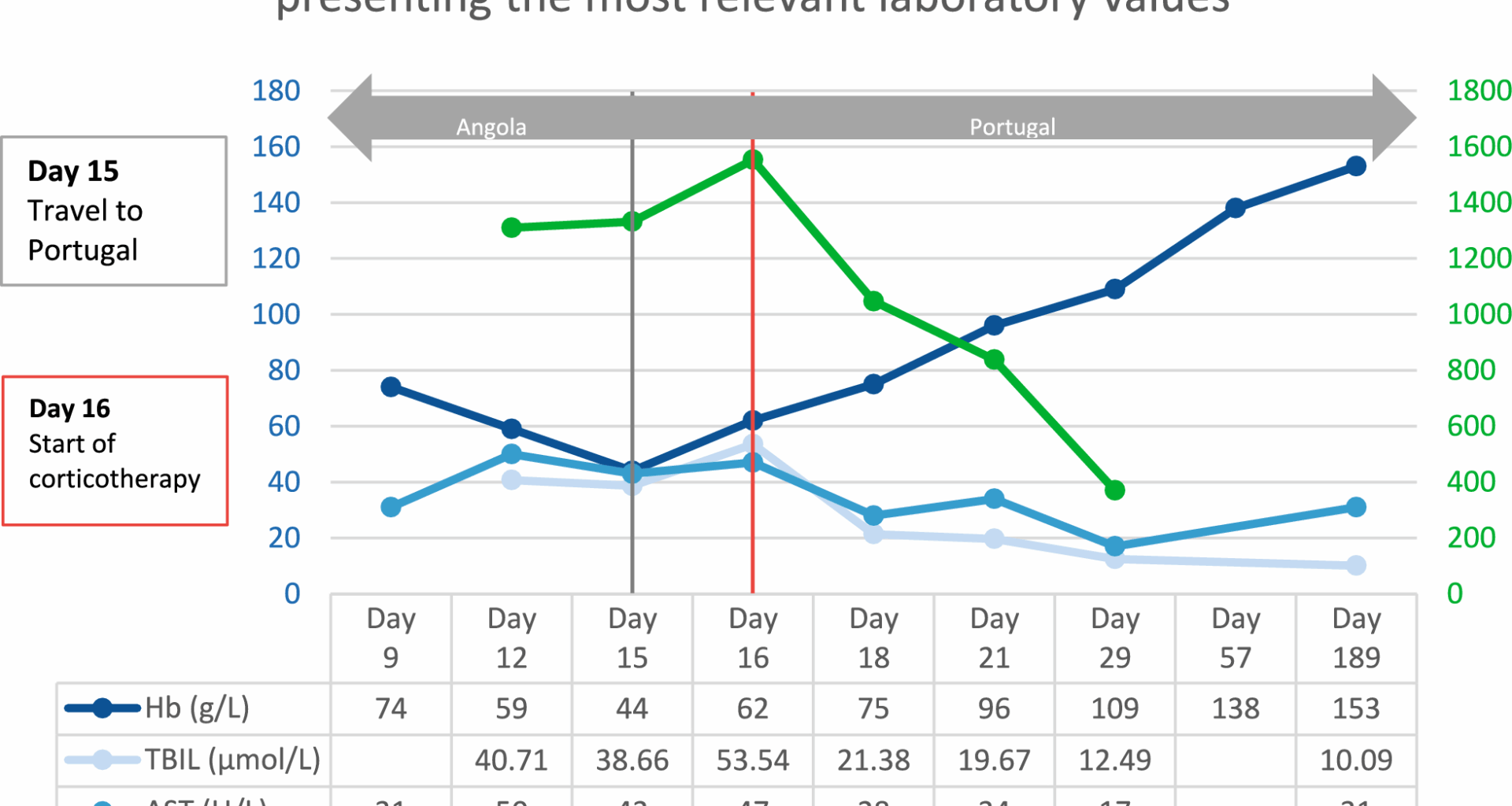
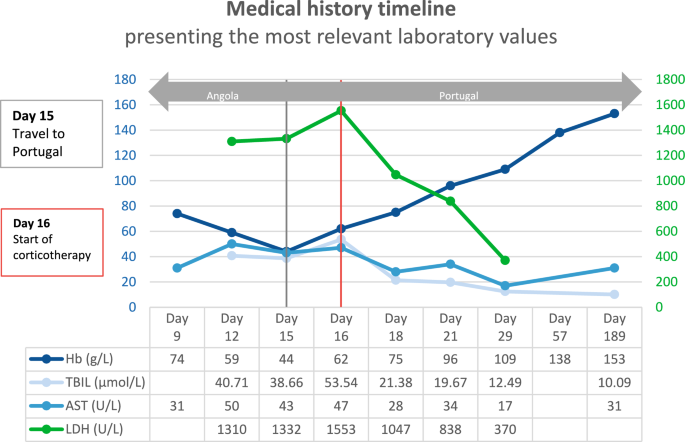
Given the clinical presentation and the laboratory finding, a presumptive diagnosis of post-artesunate delayed haemolysis (PADH) was established. The patient was admitted to the Infectious Diseases Intensive Care Unit and treatment with intravenous prednisolone was promptly initiated. During his five-day hospital stay, he received multiple blood transfusions. He was discharged with increasing levels of haemoglobin and decreasing levels of cytolytic markers (Fig. 1). Follow-up care was provided for a period of one year after the completion of corticotherapy.
The patient was hospitalized in Angola for P. falciparum infection and treatment with intravenous artesunate was initiated (Day 0). First and second follow-up in Angola (Day 9 and 12). The patient travelled to Portugal and was admitted to the Emergency Department (Day 15). Inpatient care in the Intensive Care Unit (Day 16, 18 and 21); corticotherapy was instituted on Day 16. Hospital discharge (Day 29). First and second follow-up in Portugal (Day 57 and 189). Hb, haemoglobin; TBIL total bilirubin; AST, aspartate transferase; LDH lactate dehydrogenase
Malaria and the role of artesunate
Malaria is a globally widespread disease with an estimated disease burden of 263 million cases and 597,000 deaths in 2023 [1]. The central regions of Africa are most affected, with Central and South America and Southeast Asia also showing high incidence. The infection is transmitted by Anopheles mosquito species, many of which continue to be present in Europe [2]. Throughout history, many European countries—from Italy to Finland—battled with malaria up until the Second World War [3]. The rapid economic growth of the twentieth century and the implementation of national elimination programmes led to the virtually complete eradication of malaria in Europe by the 1970s [2]. At present, the incidence in Europe is low and mainly travel-related. In 2022, a total of 6,131 cases were reported, of which 99.8% were imported [4]. This low incidence could, in theory, be affected by the steady growth in international travel and the potentially deleterious effects of climate change [2, 5]. Thirteen acquired cases were reported in the European Union in 2022 (seven in France, three in Germany, two in Spain, and one in Ireland) [6, 7]. Most of the cases were Odyssean (airport or luggage related) and caused by P. falciparum [6, 7].
The World Health Organization (WHO) Guideline for Malaria is a regularly updated collection of recommendations on vector control, prevention, case management, and surveillance [8]. In Europe, several national guidelines exist with varying recommendations [9,10,11,12]. Thanks to harmonization efforts, a joint European guideline may be produced in the near future [9].
Artemisinin is extracted from the plant Artemisia annua (quinghao in China), sweet wormwood in English speaking countries. Its discovery as an anti-malarial was the result of project 523, a military initiative led by Tu Youyou, that aimed to identify new treatment options for quinine-resistant malaria [8,9,10]. Artemether and arteether are oil-soluble methyl and ethyl ether derivatives; when given via the intramuscular route, their absorption is slow, non-uniform and thus less effective [13]. Artesunate, on the other hand, is water-soluble, allowing for intravenous administration, and has better absorption when given as an intramuscular injection [13]. These three artemisinin derivatives are metabolized into their active metabolite, dihydroartemisinin, that has a very short half-life. Artemisinin derivatives can also be administered orally. To minimize the risk of recrudescence and drug resistance, an artemisinin-based combination therapy (ACT) is recommended when taken orally [14].
Artemisinin-based drugs are highly effective against asexual stages of Plasmodium in the erythrocytic cycle (especially young trophozoites in the ring form) and against sexual stages (gametocytes in human hosts). This means that there is a quick and efficient reduction of circulating trophozoites, and a decreased chance of parasite transmission to a new vector [15]. Artemisinin-based therapy has no effect on exo-erythrocytic stages (schizonts in human hosts), and, therefore, cannot prevent relapses of infections caused by Plasmodium ovale and Plasmodium vivax [15].
In clinical trials, artesunate demonstrated superior efficacy compared to quinine. SEAQUAMAT (South East Asian Quinine Artesunate Malaria Trial) and AQUAMAT (African Quinine Artesunate Malaria Trial), were both multicentre, open-label trials involving adults and children with severe malaria randomized to receive either artesunate or quinine [16, 17]. In the SEAQUAMAT trial, the overall mortality rate in the artesunate group was 15%, compared to 22% in the quinine group. The AQUAMAT trial reported mortality rates of 8.5% in the artesunate group versus 10.9% in the quinine group. In addition to its efficacy, artesunate demonstrated a more favourable safety profile in both studies [16, 17].
Since 2006, the WHO has recommended the first-line use of a three-day course of ACT to treat uncomplicated malaria and intravenous artesunate to treat severe malaria infection [18]. In the European Union, artesunate was granted orphan drug designation in 2007 (EU/3/07/430). However, good manufacturing practice was not secured in Europe for a long time [19]. This changed in 2021, when artesunate Amivas was granted marketing authorization (EU/1/21/1582).
Post-artesunate delayed haemolysis
After the worldwide implementation of artesunate as a first-line treatment for malaria, several cases of delayed-onset haemolytic anaemia (DHA) have been reported in non-immune travellers treated with intravenous artesunate since 2011 [21,22,23,24]. The term “non-immune traveller” includes individuals that originate from non-endemic areas of any geographical location. Further investigation revealed that this adverse effect also affects children and adults with hyperparasitaemia [20,21,22]. DHA was observed not only after the administration of intravenous artemisinin derivatives, but also following the use of intramuscular, oral and even intrarectal preparations [21, 23,24,25,26]. The terms “delayed-onset haemolytic anaemia after treatment with artesunate”, “post-artesunate or post-artemisinin delayed haemolysis” and “post-treatment haemolysis” are often used interchangeably.
The first official warning about PADH was issued by the WHO in 2013, highlighting the need for further research into the pathophysiology of PADH, and encouraging physicians to set up registries to help monitor the incidence, disease progression and outcome of PADH [22]. The pathophysiology of PADH has since been—at least partially—clarified [27, 28]. Artemisinin-derivatives produce reactive oxygen species and free radicals that directly harm the parasite, especially intra-erythrocytic ring-trophozoites. Infected erythrocytes pass through the spleen’s interendothelial gaps where dead parasites are cleared by splenic macrophages, a process termed “pitting”. The once-infected erythrocytes that return to the circulation are smaller and have impaired structural integrity, resulting in a significantly reduced lifespan of approximately 7–21 days. The clearance of these once-infected erythrocytes is the underlying mechanism of DHA. This process helps explain why PADH is more frequent in patients with severe malaria with hyperparasitaemia: more ring trophozoites mean more pitted erythrocytes and thus more severe haemolysis [28]. This mechanism, however, does not explain why this side-effect only occurs in a subset of patients [26].
Jauréguiberry et al. [28] were able to detect and quantify these pitted erythrocytes using conventional fluorescence microscopy, flow cytometry, and two different markers: immunofluorescent (IF) staining to mark plasmodial antigens deposited in the RBC membrane and Hoechst dye, a nuclear DNA marker, to identify intraerythrocytic parasites [28, 29]. Uninfected erythrocytes were double negative (IF and DNA), infected erythrocytes double positive, and once-infected erythrocytes IF-positive and DNA-negative [28].
Additional mechanisms that may contribute to DHA include artemisinin-induced suppression of erythropoiesis and drug-induced haemolysis. These mechanisms could help explain case reports of haemolytic anaemia in splenectomized patients, and PADH cases with a positive direct antiglobulin test (DAT) result [21, 28, 30].
At present, there are no uniformly accepted diagnostic criteria for PADH (see Table 1). In most studies, a late-onset haemolytic episode occurring 1–4 weeks after the initiation of artesunate therapy in non-immune patients or in patients with hyperparasitaemia, after the complete clearance of asexual parasitaemia, was considered indicative of PADH [19,20,21, 26,27,28, 31, 32]. Hyperparasitaemia was defined either by WHO or local guidelines, and the diagnosis of haemolysis was mainly based on altered haemoglobin, lactate-dehydrogenase and haptoglobin levels [19,20,21, 26,27,28, 31, 32]. Due to the lack of a uniform case definition, estimating the incidence of PADH is challenging. A prospective study by Jauréguiberry et al. [31] showed a 27% incidence in a cohort of 78 patients. A large prospective study by Roussel et al. [19] found that PADH affected 42.8% of patients. Most cases seem to be mild and occur after discharge, therefore PADH incidence might be underestimated. Overall, the incidence rates vary between 7.0 and 57.9%, with higher rates amongst non-immune travellers [19,20,21, 23, 24, 26, 28, 31,32,33]. Most authors agree that patients receiving artemisinin-based therapy should be warned of PADH, and that a tight, weekly follow-up for about four weeks after treatment initiation, with clinical examination and hematological assessment is necessary [8, 21, 27, 28, 32].
Table 1 Definition of post-artesunate delayed haemolysis (PADH); LDH, lactate-dehydrogenase
PADH is usually a self-limited complication and most cases resolve spontaneously. Non-immune travellers with higher parasitaemia may experience more severe haemolytic episodes requiring intensive care and blood transfusions that are readily available in high-resource settings [19, 20, 23, 26, 28, 32]. Managing patients that withhold consent to blood transfusion, however, is a major concern. To date, only one fatal case has been directly linked to PADH and was precisely the result of the patient’s refusal of blood transfusion on religious grounds [34]. The follow-up and treatment of PADH in low-resource settings also raise critical concerns.
In about half of all PADH cases, patients present with a positive DAT that may lead to the initiation of empirical corticotherapy (mainly with prednisolone) [33, 35]. However, a multi-centre, retrospective study by Paccoud et al. [36] found that half of non-immune travellers with severe malaria have a positive DAT result, suggesting that DAT positivity may be more related to the pathophysiology of the infection itself, than to PADH.
Laboratory diagnostic challenges—differential diagnosis of PADH and malaria
Haemolysis should be suspected when a patient presents to the emergency department with some of the following signs or symptoms: fatigue, dyspnoea, tachycardia, hypotension, anaemia, jaundice, or haemoglobinuria. A wide range of inherited and acquired conditions can cause haemolytic anaemia.
With the increase of international travel, malaria and PADH are two etiologies that should be considered in the clinical decision making process. A thorough travel and medication history is essential to accurately diagnose malaria and PADH in non-immune travellers.
It is important to emphasize that despite all advances in laboratory technology, the diagnosis of malaria is still based on two simple methods: light microscopy and RDT [8, 21]. Molecular diagnostic tests can aid in the diagnosis of mixed infections and disease detection in cases of low parasite density, however, they only complement and do not substitute the former two methods in malaria management [8].
Light microscopy remains the gold standard for laboratory confirmation of malaria. It allows for a qualitative evaluation of different malaria species (P. falciparum, P. vivax, P. ovale, Plasmodium malariae, and Plasmodium knowlesi), and quantification of the parasitaemia that is essential to establish the severity of the infection and monitor treatment response. Good performance requires adequate training and ongoing maintenance of microscopy skills [8].
Numerous RDTs, approved by the WHO Malaria RDT Product Testing Programme, are available that detect the presence of specific malarial antigens [8]. The most common antigens are pan-specific-aldolase, lactate-dehydrogenase (either a pan-specific (pLDH) or a species-specific (PfLDH or PvLDH) antigen), and P. falciparum-specific histidine-rich protein 2 (PfHRP2).
It is crucial to understand which antigens are detected by the RDT used in the clinical laboratory for two key reasons. First, the antigen detected can distinguish infections caused by P. falciparum—the most virulent malaria species—from other less severe malaria infections, or mixed infections. Knowledge of the type of RDT antigen, along with its sensitivity and specificity, is essential for an accurate diagnostic evaluation. Second, different antigens exhibit varying propensities to remain in circulation after parasite clearance. The review by Dalrymple et al. [37] demonstrated that 50% of RDTs detecting PfHRP-2 are positive 15 days post-treatment, while 5% remain positive for more than a month. The study by Hosch et al. [38] reported a false-positive rate of 28.4% in RDTs (mainly PfHRP2, 97.3%) compared to molecular detection in a large cohort of patients participating in a malaria surveillance programme. It is clear that because of its persistence in circulation, PfHRP2 should not be used to evaluate treatment response [21, 39]. The pLDH antigen has a much faster clearance rate, however, still has a high false-positive rate [21, 39]. Since pLDH is also produced by asexual malaria parasites, patients with uncomplicated malaria and persisting gametocytaemia may still test positive [39].
Overall, light microscopy is still the best method to detect malaria infection and monitor treatment response. However, its diagnostic accuracy depends entirely on the expertise of the laboratory professional, emphasizing the importance of continuous training and quality assurance.
Deciding whether a patient is suffering from recrudescent infection or PADH remains challenging. Anaemia is a common feature of malarial infection and is in itself multifactorial. Erythrocytes are destroyed at schizont rupture [21, 27]. A large number of unparasitized erythrocytes suffer haemolysis because of reduced red cell deformibility, hypersplenism and possibly antibody and complement binding [21]. Bone marrow dyserythropoiesis, driven by the intramedullary production of proinflammatory cytokines, might persist for weeks following infection [21, 27]. Blackwater fever is a rare but serious complication presenting with fever, severe haemolytic anaemia, and acute renal failure [21]. Anaemia can also result from adverse drug or transfusion reactions [27].
In a research setting, the method described by Buffet et al. [29] and applied by Jauréguiberry et al. [28] can quantify once-infected, pitted erythrocytes. However, this method is too cumbersome and costly to be applied in the clinical setting [27,28,29]. Another simpler method was proposed by Ndour et al. [40]. According to their study, PfHRP2 persists predominantly in the cytoplasm and food vacuole of once-infected erythrocytes in artesunate-treated malaria patients. Using one of the commercially available RDTs (BinaxNOW Malaria), the authors correlated the presence of PfHRP2 and the occurrence of PADH. They found that a positive test result using a 1:500 diluted whole blood sample, collected on the third day of artesunate treatment, could predict the occurrence of PADH with 89% sensitivity and 73% specificity [40].
The role of the DAT in PADH remains controversial. Further research is necessary to evaluate the importance of a positive Coombs test in the pathophysiology and treatment of PADH. Once again, the role of a correct microscopic diagnosis is crucial, as the institution of immunosuppressive therapy might cause recrudescence of a poorly controlled infection.