A discovery about Saturn‘s moon Titan has challenged what scientists thought was a basic rule of chemistry.
There, in the extreme cold, some supposedly fundamentally incompatible molecules may combine to form solids that have never been seen before in the Solar System, new research suggests.
This alien matter, according to a team led by chemist Fernando Izquierdo-Ruiz of Chalmers University of Technology in Sweden, is likely to be abundant on Titan.
“These are very exciting findings that can help us understand something on a very large scale, a moon [Titan] as big as the planet Mercury,” says chemist Martin Rahm of Chalmers University of Technology.
Related: Titan Could Have An Alien Biosphere – But It Might Be Dog-Sized
Titan is a fascinating little corner of the Solar System. Its lakes of methane and hydrocarbons contain complex chemistry tantalizingly close to the prebiotic chemistry needed to spark life. That doesn’t mean life is possible there, but it does present an opportunity for understanding the conditions in which life could potentially emerge.
A particular cornerstone of prebiotic chemistry is hydrogen cyanide, which, under the right conditions, forms compounds that can become the building blocks of life, such as nucleobases and amino acids. Hydrogen cyanide is abundant on Titan.
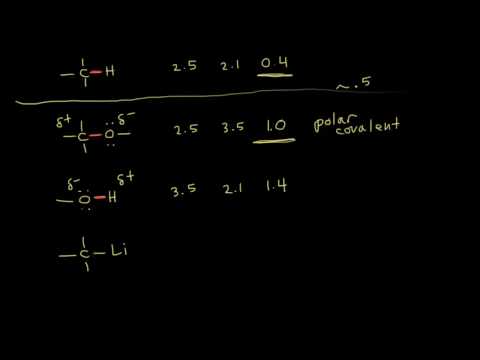 frameborder=”0″ allow=”accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share” referrerpolicy=”strict-origin-when-cross-origin” allowfullscreen>
frameborder=”0″ allow=”accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share” referrerpolicy=”strict-origin-when-cross-origin” allowfullscreen>
It’s also a strongly polar molecule, having an uneven distribution of electrons that gives it a lopsided charge.
As a rule, polar and non-polar molecules – such as the methane and ethane on Titan – tend to repel one another. It requires more energy to force them together than it does to keep them apart. This is the exact mechanism that prevents (polar) water from mixing with (nonpolar) oil.
The researchers’ investigation of hydrogen cyanide’s likely behavior on Titan started with scientists at NASA’s Jet Propulsion Laboratory trying to figure out what happens after the molecule forms in Titan’s atmosphere.
They conducted experiments at temperatures of about -180 degrees Celsius (-292 Fahrenheit), consistent with surface temperatures on Titan. At this extreme cold, hydrogen cyanide is a crystal, while methane and ethane are in liquid form.
After the experiment had run and they’d analyzed the resulting mixtures, the NASA researchers could tell something had changed, but weren’t sure what, so they recruited the chemists at Chalmers.
“This led to an exciting theoretical and experimental collaboration between Chalmers and NASA,” Rahm says. “The question we asked ourselves was a bit crazy: Can the measurements be explained by a crystal structure in which methane or ethane is mixed with hydrogen cyanide? This contradicts a rule in chemistry, ‘like dissolves like’, which basically means that it should not be possible to combine these polar and non-polar substances.”
 Infrared observations by the Cassini probe in 2015 revealed the methane lakes under Titan’s hazy atmosphere. (NASA/JPL/University of Arizona/University of Idaho)
Infrared observations by the Cassini probe in 2015 revealed the methane lakes under Titan’s hazy atmosphere. (NASA/JPL/University of Arizona/University of Idaho)
The experimental setup was similar: a chamber tuned down to a temperature of around -180 degrees Celsius, in which the researchers grew crystals of hydrogen cyanide. Into this environment, they introduced methane, ethane, propane, and butane, using Raman spectroscopy to record how the molecules vibrate.
They recorded small, but distinct, shifts in the oscillations of the hydrogen cyanide after exposure to methane and ethane – indicating that these incompatible substances were not just hanging out next to each other, but interacting.
The directions of these shifts suggested that the hydrogen bonds in the hydrogen cyanide were being subtly strengthened, bent and stretched by the methane and ethane.
Next, the team turned to computer modeling to confirm their suspicions: Methane and ethane had slipped between the gaps in the hydrogen cyanide crystal lattice, combining to form structures known as co-crystals that remain stable at Titan-like temperatures.
 frameborder=”0″ allow=”accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share” referrerpolicy=”strict-origin-when-cross-origin” allowfullscreen>
frameborder=”0″ allow=”accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share” referrerpolicy=”strict-origin-when-cross-origin” allowfullscreen>
Under Titan-like conditions, the researchers concluded, molecules don’t thermally jiggle about the way they do at higher temperatures, which allowed the methane and ethane to penetrate the hydrocyanide, showing how molecules that usually hate each other can interact and combine.
“The discovery of the unexpected interaction between these substances could affect how we understand the Titan’s geology and its strange landscapes of lakes, seas, and sand dunes,” Rahm says.
We might have to wait a few years before the significance of this bizarre chemistry can be confirmed, unfortunately, with the anticipated Dragonfly probe not expected to make landfall on Saturn’s odd moon until 2034.
“Until then, these structures represent a humbling reminder of just how surprising fundamental chemistry can be,” the researchers write.
In future work, the researchers hope to discover what other nonpolar substances might play nicely with hydrogen cyanide if conditions are just right.
The research has been published in the Proceedings of the National Academy of Sciences.

