QUICK FACTS
Milestone: Scientists develop a chemical recipe for watching molecules in living creatures
Date: Oct. 23, 2007
Where: The University of California, Berkeley and other labs
Who: A team of scientists led by Carolyn Bertozzi
In 2007, scientists published a paper that laid out a recipe for a new type of biochemistry. The method would allow scientists to see what was happening in organisms in real time.
Glycans are one of the three major classes of biomolecules (alongside proteins and nucleic acids) and had been implicated in inflammation and disease, but scientists had found them challenging to visualize. To do so, Bertozzi built upon a chemical approach pioneered by biochemists K. Barry Sharpless, of Scripps Research, and Morten Meldal, of the University of Copenhagen.
You may like
Sharpless had laid out a vision for “click chemistry” — a way to rapidly build complex biological molecules by snapping smaller subunits together.
Biological molecules often have backbones of bonded carbon atoms, but carbon atoms aren’t keen to link up. That meant that historically, chemists had to use painstaking, multistep processes that employed multiple enzymes and left unwanted byproducts. That was fine for a lab but bad for mass-producing biomolecules for pharmaceuticals.
Sharpless realized that they could simplify and scale up the process if they could snap together simple molecules that already had a complete carbon frame. They just needed a quick, powerful, reliable connector.
Separately, Sharpless and Meldal happened upon the critical connector: a chemical reaction between the compounds azide and alkyne. The trick was the addition of copper as a catalyst.
The reaction was extremely powerful and quick, and it occurred more than 99.9% of the time, without producing any byproducts.
But for Bertozzi, there was a problem: Copper is highly toxic to cells.

Carolyn Bertozzi (right) accepting a chemistry award. Her work on bioorthogonal click chemistry enabled us to better visualize living cells in action. (Image credit: BENOIT DOPPAGNE via Getty Images)
So Bertozzi combed the literature to devise click chemistry that was safe in living cells. She found the answer in decades’ old work: Azide and alkyne would react “explosively,” without the need for a catalyst, if the alkyne was forced to take on a ring shape.
You may like
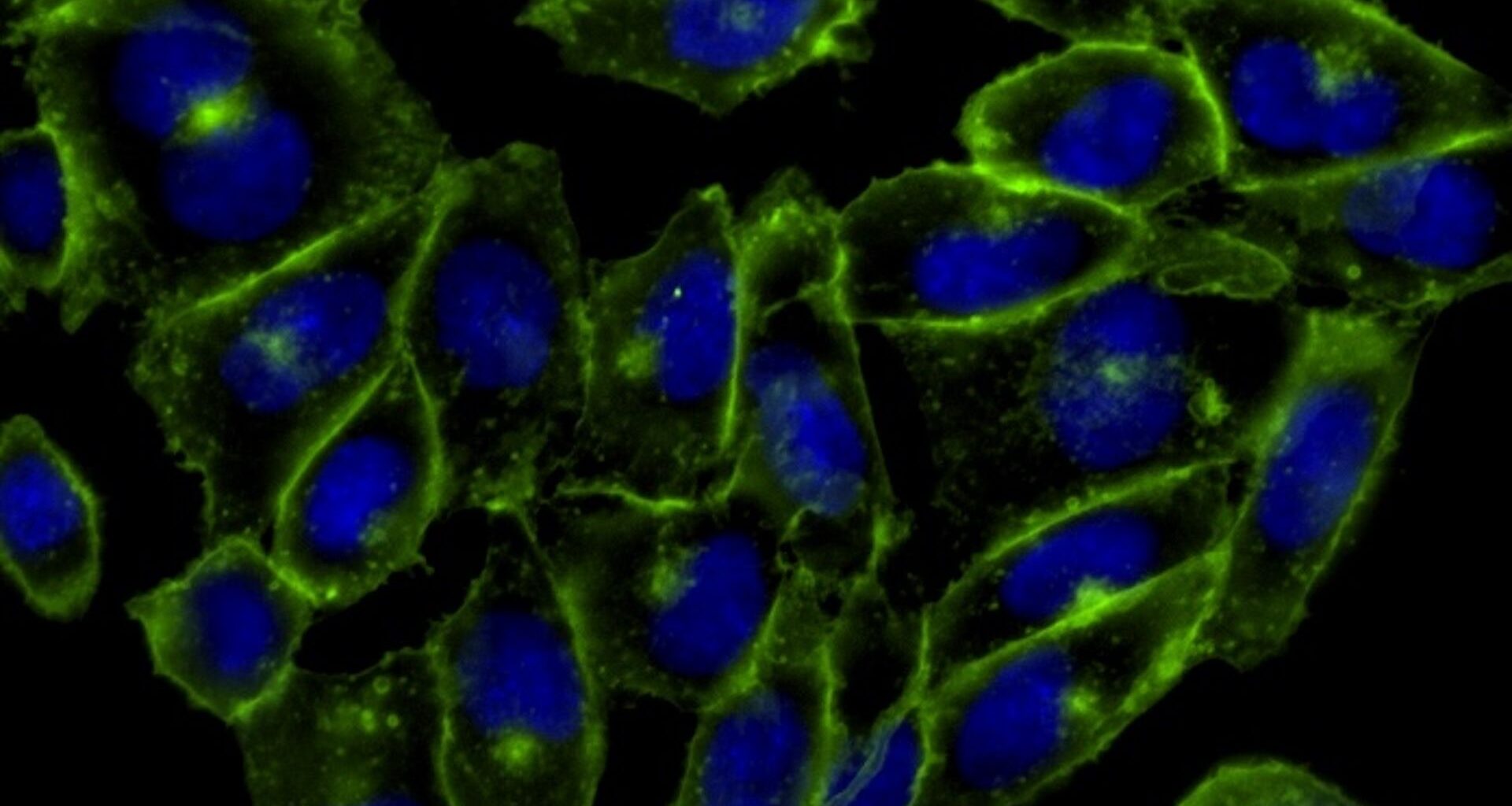
In 2004, her team demonstrated that this reaction could be used to attach azide molecules to living cells without harming them. And in 2007, Bertozzi and colleagues used her method to visualize glycans within living hamster cells.
Her process involved incorporating a carbohydrate molecule modified with azide into glycans in living cells. When they added a ring-shaped alkyne molecule that was bound to a green fluorescent protein, the azide and alkyne clicked together and the glowing green protein revealed where the glycans were in the cell.
Bertozzi dubbed the process “bioorthogonal” click chemistry — so named because it would be orthogonal to — that is, would not interfere with — the biological processes occurring in the cell. Her work has proved crucial in understanding how small molecules move through living cells. It has been used to track glycans in zebrafish embryos, to see how cancer cells mark themselves safe from immune attack using the sugar molecules, and to develop radioactive “tracers” for biomedical imaging. And click chemistry more broadly has supercharged the process of drug discovery.
In 2022, Sharpless, Meldal and Bertozzi earned the Nobel Prize in chemistry for their work on click chemistry.