Explanation for the choice of comparators {6b}
The double-blind placebo-controlled design allows for an unbiased assessment of efficacy and safety. The use of placebo is justified by the intent to assess absolute efficacy and safety of RMC-035 and is based upon absence of any approved therapeutic drug for reducing the risk of loss of kidney function in cardiac surgery patients.
Intervention description {11a}
The study intervention RMC-035 or matching placebo, also called investigational medicinal product (IMP), will be delivered by the sponsor as drug concentrate to be diluted by a pharmacist unblinded to the randomised assignment. Administration to study patients according to standard procedures will be performed by trained blinded study site staff. The pharmacist operates separately from the blinded study site team.
Administration of blinded IMP will be done using a syringe pump over a duration of approximately 60 min. Study drug should be administered via a central venous catheter (CVC) whenever possible. One lumen of the CVC should be reserved for IMP administration in order to avoid potential interactions. If a CVC is not available, IMP may also be delivered through a peripheral venous line. Detailed guidelines on the storage, dilution and administration of the study drug are provided in a Pharmacy Manual delivered to each study site.
Criteria for discontinuing or modifying allocated interventions {11b}
No dose modifications are allowed.
Criteria for permanent discontinuation of IMP include:
-
Development of stage ≥ 2 AKI, according to Kidney disease: Improving global outcomes (KDIGO) definition based on local laboratory test results.
-
Need for dialysis (continuous and intermittent)
-
Need for mechanical circulatory support (MCS) or extracorporeal membrane oxygenation (ECMO).
-
Reporting of a grade 3 (moderate intensity) or higher adverse event of infusion site reaction or infusion related reaction.
-
Abnormal liver chemistry findings in blood:
-
◦ Alanine-aminotransferase (ALT) ≥ 3 × upper limit of normal (ULN) and total bilirubin ≥ 2 × ULN without medically plausible alternative explanation
-
◦ ALT ≥ 3 × ULN if associated with symptoms (new or worsening) believed to be related to hepatitis (such as fatigue, nausea, vomiting, right upper quadrant pain or tenderness or jaundice) or believed to be related to hypersensitivity (such as fever, rash or eosinophilia)
-
◦ ALT ≥ 3 × ULN and international normalized ratio > 1.5 without medically plausible alternative explanation
-
◦ ALT ≥ 8 × ULN in any individual sample during the treatment period
-
A temporary discontinuation of IMP may be considered if, in the opinion of the investigator, a participant’s medical condition precludes dosing of study intervention due to a documented serious safety concern. In case of a temporary discontinuation of study intervention, the missed dose will not be replaced or administered at a later time point.
Strategies to improve adherence to interventions {11c}
All three doses of IMP will be administered under medical supervision in the surgery room, intensive care unit, or on the normal ward by trained study staff; therefore, no additional measures to assure adherence to the intervention protocol are required. Start and stop times of administration as well as volume administered will be documented.
Relevant concomitant care permitted or prohibited during the trial {11d}
Concomitant therapy is recorded during the study until the end-of-study (EOS) visit. Following the treatment and hospital stay, recording will be limited to relevant medication, eg antihypertensive treatments and drugs known to have an impact on renal hemodynamic, eg sodium glucose co-transporter 2 (SGLT2) inhibitors, angiotensin converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), as well as medications related to treat serious adverse events (SAEs) or given in relation to RRT. Detailed instructions on requirement for concomitant therapy collection will be provided in the case report form (CRF) completion guidelines.
Restricted medications include nephrotoxins such as non-steroidal anti-inflammatory drugs and angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARB) and sodium glucose co-transporter 2 (SGLT2) inhibitors, which all are to be discontinued before surgery and not be used during the first 72 h after the first dose of the IMP, unless strictly indicated.
Provisions for post-trial care {30}
There are no trial-specific provisions for post-trial care in place. The sponsor will ensure that participants are covered by relevant insurances during their participation in the study, in accordance with local laws and regulations. Liability for study induced injury will be covered according to local requirements.
Outcomes {12}
All endpoints involving eGFR are based on the CKD Epidemiology Collaboration (CKD-EPI) equation of 2021 using SCr assessed in a central laboratory [9]. Calculations of eGFR based on CKD-EPI equations using centrally sampled CysC [12] and a combination of SCr and CysC [9] are used for sensitivity analyses.
Primary endpoint
The primary endpoint is the change from baseline in eGFR at Day 90, comparing pooled RMC-035 dose groups to placebo.
In the clinical routine, eGFR is the gold standard for assessment of renal function. It is also an endpoint for irreversible loss of kidney function, a diagnostic marker of CKD, and on the direct path to ESRD [13, 14]. Moreover, a permanent eGFR decline has significant prognostic implications, leading to long-term adverse outcomes on individual patient health, including elevated risk for cardiovascular events, ESRD, and mortality [15,16,17].
Key secondary endpoints
Secondary endpoints of this study include change from baseline in eGFR at Day 90, MAKE90, and individual components of MAKE90. These endpoints will be assessed using both pooled analyses and dose-level comparisons against placebo.
Other secondary endpoints
Other secondary endpoints are based upon pooled, and by dose strength, comparisons of
-
Occurrence of MAKE60 and each MAKE60 component
-
Change from baseline in eGFR at Day 7 and Day 60
-
Change from baseline in SCr and CysC until Day 7
-
Occurrence of AKI based on SCr until Day 4; this includes assessment of AKI stage per KDIGO guideline [10]
-
Presence, titre, and cross-reactivity with endogenous A1M, of ADAs at baseline, Day 7, Day 60, and Day 90
-
Characteristics of ADA with regards to neutralizing activity and isotype
Exploratory endpoints
Changes from baseline in concentrations of markers of complement activation, neutrophil activation, cytokine release and mast cell activation and plasma-concentrations of RMC-035 (area under the curve [AUC], maximum concentration [Cmax] and trough levels [Ctrough]) will be assessed.
Safety endpoints
The assessment of safety includes pooled, and by dose strength, comparisons of
-
Nature, frequency and severity of treatment emergent adverse events (TEAE); a TEAE is any adverse event (AE) that occurs after start of first administration of IMP and through 72 h (inclusive) after the end of the final administration of IMP
-
Nature, frequency and severity of post-treatment AEs (PTAEs); a PTAE is any AE that occurs later than 72 h after the end of the final administration of IMP
-
Frequency and severity of TEAEs suggestive of infusion related reactions
-
Laboratory test (serum chemistry, haematology and liver function) values and changes from baseline
-
Vital signs (blood pressure, pulse rate, respiratory rate, and oxygen saturation), and electrocardiogram (ECG) values and changes from baseline
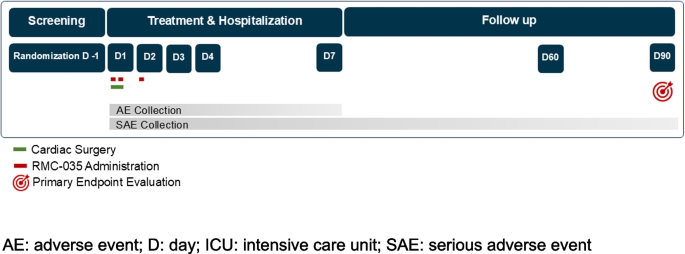
Participant timeline {13}
The study consists of 8 visits for each participant, and has three study periods:
-
Visit 1: Screening period of 30 days (Day −30 to Day −1)
-
◦ Day −1, the day before surgery, is a required study timepoint for randomisation and sampling of first baseline SCr
-
-
Visits 2 to 6: Treatment and hospital stay period of approximately 7 days (Day 1 to Day 7)
-
◦ Day 1 before the patient is prepared for surgery is the required timepoint for sampling of second baseline SCr
-
◦ Treatment with study drug occurs on Day 1 at 0 and 6 h, and Day 2 at 24 h
-
◦ Hospital stay continues until discharge at approximately Day 7
-
-
Visits 7 and 8: Follow-up period until Day 90 (from discharge to Day 90)
During the course of the study, visits and assessments will be performed as visualised in the study flow chart [Fig. 1] and as defined in the schedule of assessments for the full study period [Table 3] and for Days 1 and 2 [Table 4].
Study flowchart. AE: adverse event; D: day; ICU: intensive care unit; SAE: serious adverse event
Table 3 Schedule of assessments (Days 1 and 2)
Table 4 Schedule of assessments (full study period)Sample size {14}
A total of 161 participants will be randomised in a 2:2:3 ratio across three study arms: 46 for RMC-035 low dose, 46 for RMC-035 high dose, and 69 for placebo.
This design aims to achieve at least 80% power to detect a significant effect of pooled dose levels compared to placebo at a 10% two-sided significance level, assuming a treatment effect of 5 mL/min/1.73m2 and a standard deviation of 12 mL/min/1.73m2. The expected effect size is conservatively based on observations from the AKITA study in the subgroup with eGFR 2, where a lower starting dose was administered [8]. In this subgroup, descriptive analysis indicated a difference in eGFR of 6.5 mL/min/1.73m2, while a mixed model of repeated measures (MMRM) showed 7.9 mL/min/1.73m2. The standard deviation was derived from the AKITA study, which reported approximately 13 mL/min/1.73m2. To minimise variability, double assessment of centrally sampled serum creatinine at baseline, days 60 and 90 will be used for eGFR calculation, with an expected standard deviation of 12 mL/min/1.73m2 in the current study. A 10% drop-out rate is anticipated for the 90-day assessment, along with a 5% information loss.
Recruitment {15}
Participants will be recruited from the pool of patients being scheduled for relevant open-heart procedures at the selected study sites. Study sites were mainly selected based upon enrolment experience in the previous phase 2 AKITA study.