This retrospective cohort study provides important real-world insights into the safety of DTG-based antiretroviral regimens among Thai PLHIV. Over half of the cohort (53.86%) experienced at least one ADE within the first year of DTG therapy. This prevalence is markedly higher than the 6.30% incidence reported in a recent prospective Tanzanian cohort, which followed 935 participants for three months after DTG initiation across 25 care clinics [16]. The Tanzanian study identified mostly mild dermatologic or neuropsychiatric symptoms—such as pruritus, rash, headache, and insomnia—with few grade 3–4 events, concluding that DTG was generally well tolerated. In contrast, our study included a longer 12-month observation window and captured both clinical and laboratory-defined toxicities, including renal decline, anemia, and weight gain. Therefore, the discrepancy in ADE prevalence likely reflects differences in study design, follow-up duration, ADE definitions, and data collection methods, rather than a true regional safety difference. Nonetheless, the substantially higher ADE burden in our Thai cohort underscores the need for context-specific monitoring strategies, as short-term safety data from African or Western populations may underestimate cumulative toxicities in long-term real-world use.
The most frequent ADEs in this cohort—decline in eGFR, anemia, and weight gain—are consistent with the established safety profiles of DTG and TDF [8, 20, 21, 31, 32]. Each of these events has distinct clinical implications. Weight gain may partly reflect the return-to-health phenomenon but could also signal emerging metabolic risks associated with integrase inhibitors. Anemia was often mild and likely multifactorial, involving baseline marrow suppression, nutritional status, or prior AZT exposure. Renal decline appears primarily related to TDF rather than DTG: while DTG can cause small, reversible increases in serum creatinine via inhibition of renal tubular transporters (OCT2 and MATE1), significant eGFR reductions are more consistent with TDF-associated nephrotoxicity [33, 34].
Our multivariate analysis identified several independent predictors of ADEs. Older age (≥ 40 years) and pre-existing psychiatric comorbidity were significant risk factors, consistent with literature linking increased drug susceptibility with age [22, 23] and neuropsychiatric vulnerability to integrase inhibitors [21, 24, 25]. Critically, our study identified a novel and robust predictor: a history of cryptococcosis. This association may extend beyond being a simple marker of prior immunosuppression. We hypothesize that the profound immune dysregulation required for such an opportunistic infection could lead to persistent systemic inflammation or residual end-organ damage, thereby altering drug pharmacokinetics or heightening sensitivity to ART-related toxicities [26,27,28]. Similarly, underweight status (BMI 29,30,31,32]. Baseline renal impairment (eGFR 33, 34].
Conversely, a longer duration since duration on ART prior to DTG initiation was found to be protective. This intriguing finding requires careful interpretation as it may reflect survivor bias or treatment tolerance. This aligns with the concept of a healthy survivor effect, where individuals who have achieved long-term survival are inherently a more resilient subgroup. These survivors likely possess better long-term adherence to ART, more resilient immune systems, or fewer comorbidities, making them less susceptible to ADEs. Alternatively, long-term exposure to ART may lead to a degree of treatment tolerance in this patient group. Further research is warranted to fully characterize this resilient patient subgroup and differentiate between these potential mechanisms [35].
The strengths of this study include its large sample size and the inclusion of a comprehensive 12-month follow-up period, which is critical for observing both immediate and delayed adverse events. Crucially, the findings provide valuable real-world evidence from a setting where safety data on DTG remain sparse, thereby meaningfully contributing to the evidence base guiding DTG implementation.
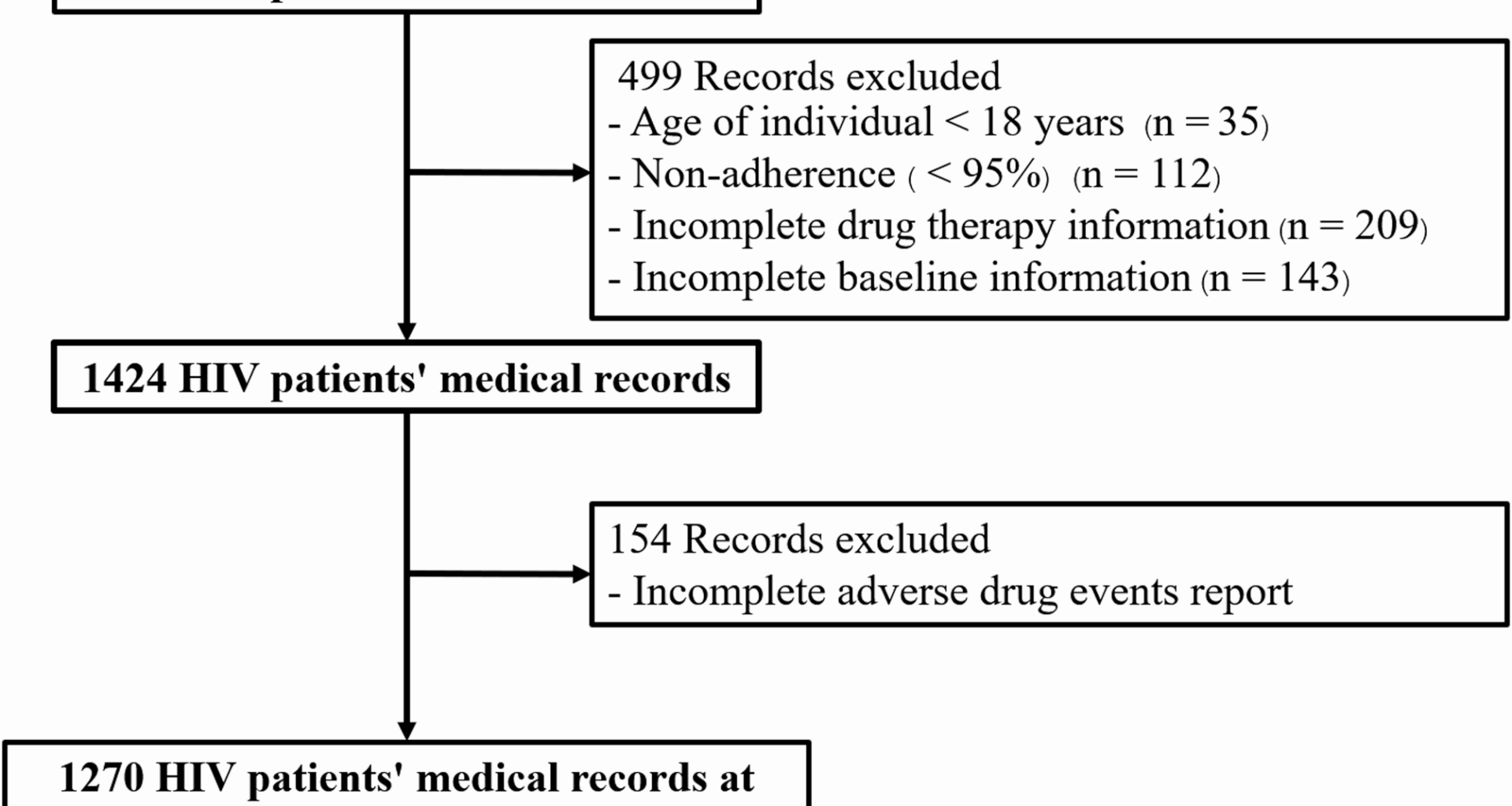
Despite these strengths, several limitations inherent to the study design and data source must be acknowledged when interpreting the results. First, while 154 records (approximately 8% of the total dataset) were excluded due to inadequate ADE documentation, this proportion was relatively modest and therefore unlikely to have introduced substantial selection bias. Second, the retrospective nature of data collection precluded the precise determination of the temporal relationship between DTG initiation and the onset of ADEs, limiting detailed temporal analyses. Similarly, reliance on existing documentation led to inconsistent recording of ADE severity grading. By protocol, we defined any documented event of Grade (per CTCAE) as an ADE, meaning that mild or clinically insignificant events were included. Consequently, this study cannot report the proportion of Grade 3–4 ADEs, which may overestimate the overall prevalence of clinically significant toxicity. Third, the study could not reliably estimate the rate of DTG discontinuation attributable to ADEs, as patients who discontinued or switched therapy before completing one year of DTG were excluded due to incomplete documentation. Fourth, data regarding historical or chronic HBV/HCV co-infection and the concomitant use of potentially nephrotoxic agents (e.g., co-trimoxazole, amphotericin B) were unavailable. These unmeasured factors may act as significant confounders, particularly when evaluating renal outcomes. Fifth, because all participants received the fixed-dose combination of DTG + 3TC + TDF, it is impossible to attribute observed renal impairment to a single drug definitively. While mild, reversible increases in serum creatinine are compatible with DTG’s transporter inhibition, larger eGFR declines likely reflect TDF toxicity. Finally, consistent with the challenges of any retrospective chart review, ADEs dependent on subjective symptoms—such as insomnia or anxiety—were likely under-reported. To mitigate under-ascertainment, we included only participants with complete one-year follow-up and documented ADE data; nevertheless, a prospective multicenter design with standardized reporting and Patient-Reported Outcome Measures (PROMs) is necessary to provide more complete safety data.
Ultimately, our findings have immediate clinical implications. They call for a shift towards more individualized patient assessment and proactive, targeted monitoring. Enhanced vigilance is particularly crucial for high-risk groups: older patients, those with psychiatric disorders, a history of severe opportunistic infections like cryptococcosis, underweight status, or baseline renal impairment. Tailored management for these vulnerable individuals is essential to enhance patient safety and optimize long-term outcomes.