Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577(7791):561–5.
Patil NS, Nabet BY, Müller S, Koeppen H, Zou W, Giltnane J, et al. Intratumoral plasma cells predict outcomes to PD-L1 Blockade in non-small cell lung cancer. Cancer Cell. 2022;40(3):289–e3004.
Chong X, Madeti Y, Cai J, Li W, Cong L, Lu J, et al. Recent developments in immunotherapy for gastrointestinal tract cancers. J Hematol Oncol. 2024;17(1):65.
Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for early Triple-Negative breast cancer. N Engl J Med. 2020;382(9):810–21.
Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. In: Aster JC, Feany MB, editors. Annual Review of Pathology: Mechanisms of Disease, Vol 16, 2021. Annual Review of Pathology-Mechanisms of Disease. 162021. pp. 223 – 49.
Ma W, Xue R, Zhu Z, Farrukh H, Song W, Li T, et al. Increasing cure rates of solid tumors by immune checkpoint inhibitors. Exp Hematol Oncol. 2023;12(1):10.
Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. 2023;22(1):40.
Sharma P, Goswami S, Raychaudhuri D, Siddiqui BA, Singh P, Nagarajan A, et al. Immune checkpoint therapy-current perspectives and future directions. Cell. 2023;186(8):1652–69.
Yin Y, Feng W, Chen J, Chen X, Wang G, Wang S, et al. Immunosuppressive tumor microenvironment in the progression, metastasis, and therapy of hepatocellular carcinoma: from bench to bedside. Exp Hematol Oncol. 2024;13(1):72.
Shen KY, Zhu Y, Xie SZ, Qin LX. Immunosuppressive tumor microenvironment and immunotherapy of hepatocellular carcinoma: current status and prospectives. J Hematol Oncol. 2024;17(1):25.
Schumacher TN, Thommen DS. Tertiary lymphoid structures in cancer. Science. 2022;375(6576):eabf9419.
Laumont CM, Banville AC, Gilardi M, Hollern DP, Nelson BH. Tumour-infiltrating B cells: immunological mechanisms, clinical impact and therapeutic opportunities. Nat Rev Cancer. 2022;22(7):414–30.
Sato Y, Silina K, van den Broek M, Hirahara K, Yanagita M. The roles of tertiary lymphoid structures in chronic diseases. Nat Rev Nephrol. 2023;19(8):525–37.
Radandish M, Mashhadi N, Aghayan AH, Taghizadeh M, Salehianfard S, Yahyazadeh S, et al. In-depth insight into tumor-infiltrating stromal cells linked to tertiary lymphoid structures and their prospective function in cancer immunotherapy. Exp Hematol Oncol. 2025;14(1):105.
Gutiérrez-Melo N, Baumjohann D. T follicular helper cells in cancer. Trends Cancer. 2023;9(4):309–25.
Liu W, You W, Lan Z, Ren Y, Gao S, Li S, et al. An immune cell map of human lung adenocarcinoma development reveals an anti-tumoral role of the Tfh-dependent tertiary lymphoid structure. Cell Rep Med. 2024;5(3):101448.
Shiao SL, Gouin KH 3rd, Ing N, Ho A, Basho R, Shah A, et al. Single-cell and Spatial profiling identify three response trajectories to pembrolizumab and radiation therapy in triple negative breast cancer. Cancer Cell. 2024;42(1):70–e848.
Künzli M, Masopust D. CD4(+) T cell memory. Nat Immunol. 2023;24(6):903–14.
Lo Tartaro D, Aramini B, Masciale V, Paschalidis N, Lofaro FD, Neroni A, et al. Metabolically activated and highly polyfunctional intratumoral VISTA(+) regulatory B cells are associated with tumor recurrence in early-stage NSCLC. Mol Cancer. 2025;24(1):16.
Xiao X, Lao XM, Chen MM, Liu RX, Wei Y, Ouyang FZ, et al. PD-1hi identifies a novel regulatory B-cell population in human hepatoma that promotes disease progression. Cancer Discov. 2016;6(5):546–59.
Shalapour S, Font-Burgada J, Di Caro G, Zhong Z, Sanchez-Lopez E, Dhar D, et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521(7550):94–8.
Wang H, Li J, Wang Y, Chen Y, Zhang W, Pan X, et al. IgG4-mediated M2 macrophage polarization in tertiary lymphoid structures of esophageal cancer: implications for immunosuppression. Front Immunol. 2024;15:1497783.
Groen-van Schooten TS, Franco Fernandez R, van Grieken NCT, Bos EN, Seidel J, Saris J, et al. Mapping the complexity and diversity of tertiary lymphoid structures in primary and peritoneal metastatic gastric cancer. J Immunother Cancer. 2024;12(7):e009243.
Meylan M, Petitprez F, Lacroix L, Di Tommaso L, Roncalli M, Bougoüin A, et al. Early hepatic lesions display immature tertiary lymphoid structures and show elevated expression of immune inhibitory and immunosuppressive molecules. Clin Cancer Res. 2020;26(16):4381–9.
Gulati GS, D’Silva JP, Liu Y, Wang L, Newman AM. Profiling cell identity and tissue architecture with single-cell and spatial transcriptomics. Nat Rev Mol Cell Biol. 2025;26(1):11–31.
Rao A, Barkley D, França GS, Yanai I. Exploring tissue architecture using spatial transcriptomics. Nature. 2021;596(7871):211–20.
Tang Z, Bai Y, Fang Q, Yuan Y, Zeng Q, Chen S, et al. Spatial transcriptomics reveals Tryptophan metabolism restricting maturation of intratumoral tertiary lymphoid structures. Cancer Cell. 2025;43(6):1025–e4414.
Wang S, Wang H, Li C, Liu B, He S, Tu C. Tertiary lymphoid structures in cancer: immune mechanisms and clinical implications. MedComm. 2024;5(3):e489.
Yang J, Xiong X, Zheng W, Xu H, Liao X, Wei Q, et al. The roles of tertiary lymphoid structures in genitourinary cancers: molecular mechanisms, therapeutic strategies, and clinical applications. Int J Surg. 2024;110(8):5007–21.
Zhao L, Jin S, Wang S, Zhang Z, Wang X, Chen Z, et al. Tertiary lymphoid structures in diseases: immune mechanisms and therapeutic advances. Signal Transduct Target Ther. 2024;9(1):225.
Kinker GS, Vitiello GAF, Diniz AB, Cabral-Piccin MP, Pereira PHB, Carvalho MLR, et al. Mature tertiary lymphoid structures are key niches of tumour-specific immune responses in pancreatic ductal adenocarcinomas. Gut. 2023;72(10):1927–41.
Johansson-Percival A, Ganss R. Therapeutic induction of tertiary lymphoid structures in cancer through stromal remodeling. Front Immunol. 2021;12:674375.
Rodriguez AB, Peske JD, Woods AN, Leick KM, Mauldin IS, Meneveau MO, et al. Immune mechanisms orchestrate tertiary lymphoid structures in tumors via cancer-associated fibroblasts. Cell Rep. 2021;36(3):109422.
Wang MJ, Zhang HL, Chen F, Guo XJ, Liu QG, Hou J. The double-edged effects of IL-6 in liver regeneration, aging, inflammation, and diseases. Exp Hematol Oncol. 2024;13(1):62.
Workel HH, Lubbers JM, Arnold R, Prins TM, van der Vlies P, de Lange K, et al. A transcriptionally distinct CXCL13(+)CD103(+)CD8(+) T-cell population is associated with B-cell recruitment and neoantigen load in human cancer. Cancer Immunol Res. 2019;7(5):784–96.
Reste M, Ajazi K, Sayi-Yazgan A, Jankovic R, Bufan B, Brandau S, et al. The role of dendritic cells in tertiary lymphoid structures: implications in cancer and autoimmune diseases. Front Immunol. 2024;15:1439413.
Amisaki M, Zebboudj A, Yano H, Zhang SL, Payne G, Chandra AK, et al. IL-33-activated ILC2s induce tertiary lymphoid structures in pancreatic cancer. Nature. 2025;638(8052):1076–84.
Jenkins BH, Tracy I, Rodrigues M, Smith MJL, Martinez BR, Edmond M, et al. Single cell and spatial analysis of immune-hot and immune-cold tumours identifies fibroblast subtypes associated with distinct immunological niches and positive immunotherapy response. Mol Cancer. 2025;24(1):3.
Peske JD, Thompson ED, Gemta L, Baylis RA, Fu YX, Engelhard VH. Effector lymphocyte-induced lymph node-like vasculature enables naive T-cell entry into tumours and enhanced anti-tumour immunity. Nat Commun. 2015;6:7114.
Shen S, Cui Y, Li M, Yu K, Zhu Q, Zhang X, et al. Toll-like receptor agonists promote the formation of tertiary lymphoid structure and improve anti-glioma immunity. Neuro Oncol. 2025;27(1):140–54.
Chaurio RA, Anadon CM, Lee Costich T, Payne KK, Biswas S, Harro CM, et al. TGF-β-mediated Silencing of genomic organizer SATB1 promotes Tfh cell differentiation and formation of intra-tumoral tertiary lymphoid structures. Immunity. 2022;55(1):115–e289.
Vondenhoff MF, Greuter M, Goverse G, Elewaut D, Dewint P, Ware CF, et al. LTbetaR signaling induces cytokine expression and up-regulates lymphangiogenic factors in lymph node anlagen. J Immunol. 2009;182(9):5439–45.
Onder L, Mörbe U, Pikor N, Novkovic M, Cheng HW, Hehlgans T, et al. Lymphatic endothelial cells control initiation of lymph node organogenesis. Immunity. 2017;47(1):80–e924.
Shu DH, Sidiropoulos DN. Maturation of tertiary lymphoid structures. Methods Mol Biol. 2025;2864:43–55.
Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 2012;33(6):297–305.
An D, Chen G, Cheng WY, Mohrs K, Adler C, Gupta NT, et al. Ltβr agonism promotes antitumor immune responses via modulation of the tumor microenvironment. Cancer Res. 2024;84(23):3984–4001.
Engelhard VH, Rodriguez AB, Mauldin IS, Woods AN, Peske JD, Slingluff CL Jr. Immune cell infiltration and tertiary lymphoid structures as determinants of antitumor immunity. J Immunol. 2018;200(2):432–42.
Fleig S, Kapanadze T, Bernier-Latmani J, Lill JK, Wyss T, Gamrekelashvili J, et al. Loss of vascular endothelial Notch signaling promotes spontaneous formation of tertiary lymphoid structures. Nat Commun. 2022;13(1):2022.
Manzo A, Paoletti S, Carulli M, Blades MC, Barone F, Yanni G, et al. Systematic microanatomical analysis of CXCL13 and CCL21 in situ production and progressive lymphoid organization in rheumatoid synovitis. Eur J Immunol. 2005;35(5):1347–59.
Rouanne M, Arpaia N, Marabelle A. CXCL13 shapes tertiary lymphoid structures and promotes response to immunotherapy in bladder cancer. Eur J Cancer. 2021;151:245–8.
Johansson-Percival A, He B, Li ZJ, Kjellén A, Russell K, Li J, et al. De novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors. Nat Immunol. 2017;18(11):1207–17.
de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, et al. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011;71(20):6391–9.
Nayar S, Campos J, Chung MM, Navarro-Núñez L, Chachlani M, Steinthal N, et al. Bimodal expansion of the lymphatic vessels is regulated by the sequential expression of IL-7 and lymphotoxin α1β2 in newly formed tertiary lymphoid structures. J Immunol. 2016;197(5):1957–67.
Onder L, Papadopoulou C, Lütge A, Cheng HW, Lütge M, Perez-Shibayama C, et al. Fibroblastic reticular cells generate protective intratumoral T cell environments in lung cancer. Cell. 2025;188(2):430–. – 46.e20.
Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol. 2011;29:621–63.
Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173(1):68–78.
Liu X, Lu H, Chen T, Nallaparaju KC, Yan X, Tanaka S, et al. Genome-wide analysis identifies Bcl6-controlled regulatory networks during T follicular helper cell differentiation. Cell Rep. 2016;14(7):1735–47.
Ninomiya T, Kemmotsu N, Mukohara F, Magari M, Miyamoto A, Ueda Y, et al. Myeloid cells induce infiltration and activation of B cells and CD4 + T follicular helper cells to sensitize brain metastases to combination immunotherapy. Cancer Res. 2025;85(6):1082–96.
Song J, Wei R, Liu C, Zhao Z, Liu X, Wang Y, et al. Antigen-presenting cancer associated fibroblasts enhance antitumor immunity and predict immunotherapy response. Nat Commun. 2025;16(1):2175.
Weinstein JS, Herman EI, Lainez B, Licona-Limón P, Esplugues E, Flavell R, et al. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol. 2016;17(10):1197–205.
Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29(1):127–37.
Stewart I, Radtke D, Phillips B, McGowan SJ, Bannard O, Germinal Center B. Cells replace their antigen receptors in dark zones and fail light zone entry when Immunoglobulin gene mutations are damaging. Immunity. 2018;49(3):477–e897.
Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143(4):592–605.
Meylan M, Petitprez F, Becht E, Bougoüin A, Pupier G, Calvez A, et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity. 2022;55(3):527–e415.
Playoust E, Remark R, Vivier E, Milpied P. Germinal center-dependent and -independent immune responses of tumor-infiltrating B cells in human cancers. Cell Mol Immunol. 2023;20(9):1040–50.
Giles JR, Globig AM, Kaech SM, Wherry EJ. CD8(+) T cells in the cancer-immunity cycle. Immunity. 2023;56(10):2231–53.
St Paul M, Ohashi PS. The roles of CD8(+) T cell subsets in antitumor immunity. Trends Cell Biol. 2020;30(9):695–704.
Gebhardt T, Park SL, Parish IA. Stem-like exhausted and memory CD8(+) T cells in cancer. Nat Rev Cancer. 2023;23(11):780–98.
Kallies A, Zehn D, Utzschneider DT. Precursor exhausted T cells: key to successful immunotherapy? Nat Rev Immunol. 2020;20(2):128–36.
Hashimoto M, Kamphorst AO, Im SJ, Kissick HT, Pillai RN, Ramalingam SS, et al. CD8 T cell exhaustion in chronic infection and cancer: opportunities for interventions. Annu Rev Med. 2018;69:301–18.
Zehn D, Thimme R, Lugli E, de Almeida GP, Oxenius A. Stem-like’ precursors are the fount to sustain persistent CD8(+) T cell responses. Nat Immunol. 2022;23(6):836–47.
Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31(1):137–61.
Sykulev Y. Factors contributing to the potency of CD8(+) T cells. Trends Immunol. 2023;44(9):693–700.
Speiser DE, Chijioke O, Schaeuble K, Münz C. CD4(+) T cells in cancer. Nat Cancer. 2023;4(3):317–29.
Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, et al. CD4⁺ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013;123(7):2873–92.
Crotty ST. Follicular helper. Cell biology: a decade of discovery and diseases. Immunity. 2019;50(5):1132–48.
Knochelmann HM, Dwyer CJ, Bailey SR, Amaya SM, Elston DM, Mazza-McCrann JM, et al. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell Mol Immunol. 2018;15(5):458–69.
Li S, Na R, Li X, Zhang Y, Zheng T. Targeting interleukin-17 enhances tumor response to immune checkpoint inhibitors in colorectal cancer. Biochimica et Biophysica Acta (BBA). 2022;1877(4):188758.
Savage PA, Klawon DEJ, Miller CH. Regulatory t cell development. Annu Rev Immunol. 2020;38:421–53.
Wing JB, Tanaka A, Sakaguchi S. Human FOXP3(+) regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity. 2019;50(2):302–16.
Downs-Canner SM, Meier J, Vincent BG, Serody JS. B cell function in the tumor microenvironment. Annu Rev Immunol. 2022;40:169–93.
Engelhard V, Conejo-Garcia JR, Ahmed R, Nelson BH, Willard-Gallo K, Bruno TC, et al. B cells and cancer. Cancer Cell. 2021;39(10):1293–6.
De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nat Rev Immunol. 2015;15(3):137–48.
Seifert M, Küppers R. Human memory B cells. Leukemia. 2016;30(12):2283–92.
Sharonov GV, Serebrovskaya EO, Yuzhakova DV, Britanova OV, Chudakov DM. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat Rev Immunol. 2020;20(5):294–307.
Rosser EC, Mauri C, Regulatory. B cells: origin, phenotype, and function. Immunity. 2015;42(4):607–12.
Gao X, Cockburn IA. The development and function of CD11c(+) atypical B cells – insights from single cell analysis. Front Immunol. 2022;13:979060.
Perez CR, De Palma M. Engineering dendritic cell vaccines to improve cancer immunotherapy. Nat Commun. 2019;10(1):5408.
Guilliams M, Mildner A, Yona S. Developmental and functional heterogeneity of monocytes. Immunity. 2018;49(4):595–613.
Vincent FB, Saulep-Easton D, Figgett WA, Fairfax KA, Mackay F. The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev. 2013;24(3):203–15.
Li M, Yang Y, Xiong L, Jiang P, Wang J, Li C. Metabolism, metabolites, and macrophages in cancer. J Hematol Oncol. 2023;16(1):80.
Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416.
Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 2020;15:123–47.
Hegde S, Leader AM, Merad M. MDSC: Markers, development, states, and unaddressed complexity. Immunity. 2021;54(5):875–84.
van Vlerken-Ysla L, Tyurina YY, Kagan VE, Gabrilovich DI. Functional states of myeloid cells in cancer. Cancer Cell. 2023;41(3):490–504.
De Martin A, Stanossek Y, Pikor NB, Ludewig B. Protective fibroblastic niches in secondary lymphoid organs. J Exp Med. 2024;221(1):e20221220.
Fletcher AL, Acton SE, Knoblich K. Lymph node fibroblastic reticular cells in health and disease. Nat Rev Immunol. 2015;15(6):350–61.
Kennel KB, Bozlar M, De Valk AF, Greten FR. Cancer-associated fibroblasts in inflammation and antitumor immunity. Clin Cancer Res. 2023;29(6):1009–16.
Tsoumakidou M. The advent of immune stimulating CAFs in cancer. Nat Rev Cancer. 2023;23(4):258–69.
Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 2019;9(8):1102–23.
Krimpenfort LT, Degn SE, Heesters BA. The follicular dendritic cell: at the germinal center of autoimmunity? Cell Rep. 2024;43(3):113869.
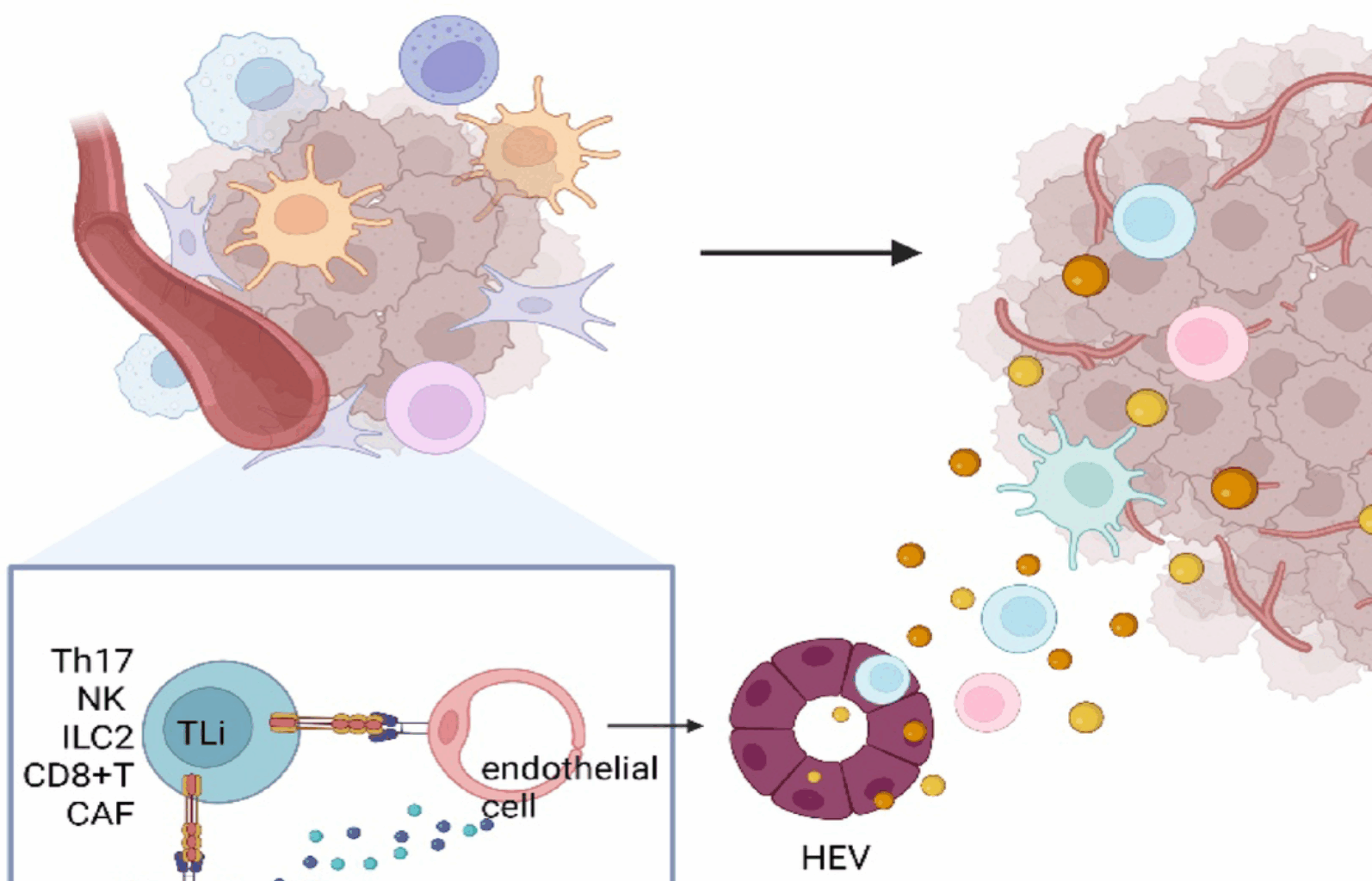
Vella G, Hua Y, Bergers G. High endothelial venules in cancer: regulation, function, and therapeutic implication. Cancer Cell. 2023;41(3):527–45.
Liu M, Li L, Cao L, Li W, Gu X, Yang M, et al. Targeted delivery of CCL3 reprograms macrophage antigen presentation and enhances the efficacy of immune checkpoint blockade therapy in hepatocellular carcinoma. J Immunother Cancer. 2025;13(2):e010947.
Zhao R, Zhang J, Ma J, Qu Y, Yang Z, Yin Z, et al. cGAS-activated endothelial cell-T cell cross-talk initiates tertiary lymphoid structure formation. Sci Immunol. 2024;9(98):eadk2612.
Wortzel I, Seo Y, Akano I, Shaashua L, Tobias GC, Hebert J, et al. Unique structural configuration of EV-DNA primes Kupffer cell-mediated antitumor immunity to prevent metastatic progression. Nat Cancer. 2024;5(12):1815–33.
Xu Z, Liu L, Jiang W, Qiu Y, Zhang B, Cheng J, et al. VHL missense mutation delineate aggressive clear cell renal cell carcinoma subtype with favorable immunotherapeutic response. J Immunother Cancer. 2024;12(10):e009963.
Mollaoglu G, Tepper A, Falcomatà C, Potak HT, Pia L, Amabile A, et al. Ovarian cancer-derived IL-4 promotes immunotherapy resistance. Cell. 2024;187(26):7492–e51022.
Haniuda K, Fukao S, Kitamura D. Metabolic reprogramming induces germinal center B cell differentiation through Bcl6 locus remodeling. Cell Rep. 2020;33(5):108333.
Jiao M, Guo Y, Zhang H, Wen H, Chen P, Wang Z, et al. ACAT1 regulates tertiary lymphoid structures and correlates with immunotherapy response in non-small cell lung cancer. J Clin Invest. 2025;135(7):e181517.
Ma J, Wu Y, Ma L, Yang X, Zhang T, Song G, et al. A blueprint for tumor-infiltrating B cells across human cancers. Science. 2024;384(6695):eadj4857.
Rodriguez AB, Engelhard VH. Insights into tumor-associated tertiary lymphoid structures: novel targets for antitumor immunity and cancer immunotherapy. Cancer Immunol Res. 2020;8(11):1338–45.
Sl NJJT, Gt N. Tertiary lymphoid structures and B lymphocytes in cancer prognosis and response to immunotherapies. Oncoimmunology. 2021;10(1):1900508.
MacFawn IP, Magnon G, Gorecki G, Kunning S, Rashid R, Kaiza ME, et al. The activity of tertiary lymphoid structures in high grade serous ovarian cancer is governed by site, stroma, and cellular interactions. Cancer Cell. 2024;42(11):1864–e815.
Yoffe L, Bhinder B, Kang SW, Zhang H, Singh A, Ravichandran H et al. Acquisition of discrete immune suppressive barriers contributes to the initiation and progression of preinvasive to invasive human lung cancer. BioRxiv. 2025.
Yan Y, Sun D, Hu J, Chen Y, Sun L, Yu H, et al. Multi-omic profiling highlights factors associated with resistance to immuno-chemotherapy in non-small-cell lung cancer. Nat Genet. 2025;57(1):126–39.
Xu AM, Haro M, Walts AE, Hu Y, John J, Karlan BY, et al. Spatiotemporal architecture of immune cells and cancer-associated fibroblasts in high-grade serous ovarian carcinoma. Sci Adv. 2024;10(16):eadk8805.
Liu X, Duda DG. Involuted TLS as a harbinger of HCC regression. Nat Immunol. 2024;25(11):1986–7.
Shu DH, Ho WJ, Kagohara LT, Girgis A, Shin SM, Danilova L, et al. Immunotherapy response induces divergent tertiary lymphoid structure morphologies in hepatocellular carcinoma. Nat Immunol. 2024;25(11):2110–23.
Xu W, Lu J, Tian X, Ye S, Wei S, Wang J, et al. Unveiling the impact of tertiary lymphoid structures on immunotherapeutic responses of clear cell renal cell carcinoma. MedComm. 2024;5(1):e461.
Le Rochais M, Hémon P, Ben-Guigui D, Garaud S, Le Dantec C, Pers JO, et al. Deciphering the maturation of tertiary lymphoid structures in cancer and inflammatory diseases of the digestive tract using imaging mass cytometry. Front Immunol. 2023;14:1147480.
Fridman WH, Meylan M, Pupier G, Calvez A, Hernandez I, Sautès-Fridman C. Tertiary lymphoid structures and B cells: an intratumoral immunity cycle. Immunity. 2023;56(10):2254–69.
Calderaro J, Petitprez F, Becht E, Laurent A, Hirsch TZ, Rousseau B, et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J Hepatol. 2019;70(1):58–65.
Xu W, Lu J, Liu WR, Anwaier A, Wu Y, Tian X, et al. Heterogeneity in tertiary lymphoid structures predicts distinct prognosis and immune microenvironment characterizations of clear cell renal cell carcinoma. J Immunother Cancer. 2023;11(12):e006667.
Zubkova E, Kalinin A, Beloglazova I, Kurilina E, Menshikov M, Parfyonova Y, et al. Artificial tertiary lymphoid structures: exploring mesenchymal stromal cells as a platform for immune niche formation. Int J Mol Sci. 2024;25(24):13286.
Teillaud JL, Houel A, Panouillot M, Riffard C, Dieu-Nosjean MC. Tertiary lymphoid structures in anticancer immunity. Nat Rev Cancer. 2024;24(9):629–46.
Fang Q, Chen S, Chen X, Zou W, Chen D, Huang Y, et al. Mature tertiary lymphoid structure associated CD103 + CD8 + Trm cells determined improved anti-tumor immune in breast cancer. Front Oncol. 2025;15:1480461.
Lynch KT, Young SJ, Meneveau MO, Wages NA, Engelhard VH, Slingluff CL Jr., et al. Heterogeneity in tertiary lymphoid structure B-cells correlates with patient survival in metastatic melanoma. J Immunother Cancer. 2021;9(6):e002273.
Joshi NS, Akama-Garren EH, Lu Y, Lee DY, Chang GP, Li A, et al. Regulatory t cells in tumor-associated tertiary lymphoid structures suppress anti-tumor t cell responses. Immunity. 2015;43(3):579–90.
Ding GY, Ma JQ, Yun JP, Chen X, Ling Y, Zhang S, et al. Distribution and density of tertiary lymphoid structures predict clinical outcome in intrahepatic cholangiocarcinoma. J Hepatol. 2022;76(3):608–18.
Zhang Y, Liu G, Zeng Q, Wu W, Lei K, Zhang C, et al. CCL19-producing fibroblasts promote tertiary lymphoid structure formation enhancing anti-tumor IgG response in colorectal cancer liver metastasis. Cancer Cell. 2024;42(8):1370–e859.
Zhang C, Wang XY, Zuo JL, Wang XF, Feng XW, Zhang B, et al. Localization and density of tertiary lymphoid structures associate with molecular subtype and clinical outcome in colorectal cancer liver metastases. J Immunother Cancer. 2023;11(2):e006425.
Lin JR, Wang S, Coy S, Chen YA, Yapp C, Tyler M, et al. Multiplexed 3D atlas of state transitions and immune interaction in colorectal cancer. Cell. 2023;186(2):363–. – 81.e19.
Xie Y, Peng H, Hu Y, Jia K, Yuan J, Liu D, et al. Immune microenvironment spatial landscapes of tertiary lymphoid structures in gastric cancer. BMC Med. 2025;23(1):59.
Ng KW, Boumelha J, Enfield KSS, Almagro J, Cha H, Pich O, et al. Antibodies against endogenous retroviruses promote lung cancer immunotherapy. Nature. 2023;616(7957):563–73.
Fridman WH, Sibéril S, Pupier G, Soussan S, Sautès-Fridman C. Activation of B cells in tertiary lymphoid structures in cancer: anti-tumor or anti-self? Semin Immunol. 2023;65:101703.
Montfort A, Pearce O, Maniati E, Vincent BG, Bixby L, Böhm S, et al. A strong B-cell response is part of the immune landscape in human High-Grade serous ovarian metastases. Clin Cancer Res. 2017;23(1):250–62.
Freitas Monteiro M, Papaserafeim M, Réal A, Puga Yung GL, Seebach JD. Anti-CD20 rituximab IgG1, IgG3, and IgG4 but not IgG2 subclass trigger Ca(2+) mobilization and cytotoxicity in human NK cells. J Leukoc Biol. 2020;108(4):1409–23.
Hu X, Zhang J, Wang J, Fu J, Li T, Zheng X, et al. Landscape of B cell immunity and related immune evasion in human cancers. Nat Genet. 2019;51(3):560–7.
Dixon KJ, Wu J, Walcheck B. Engineering anti-tumor monoclonal antibodies and Fc receptors to enhance ADCC by human NK cells. Cancers (Basel). 2021;13(2):312.
Mazor RD, Nathan N, Gilboa A, Stoler-Barak L, Moss L, Solomonov I, et al. Tumor-reactive antibodies evolve from non-binding and autoreactive precursors. Cell. 2022;185(7):1208–e2221.
Saul L, Ilieva KM, Bax HJ, Karagiannis P, Correa I, Rodriguez-Hernandez I, et al. Igg subclass switching and clonal expansion in cutaneous melanoma and normal skin. Sci Rep. 2016;6:29736.
De Monte L, Clemente F, Ruggiero E, Pini R, Ceraolo MG, Schiavo Lena M, et al. Pro-tumor Tfh2 cells induce detrimental IgG4 production and PGE(2)-dependent IgE inhibition in pancreatic cancer. EBioMedicine. 2023;97:104819.
Jordakieva G, Bianchini R, Reichhold D, Piehslinger J, Groschopf A, Jensen SA, et al. IgG4 induces tolerogenic M2-like macrophages and correlates with disease progression in colon cancer. Oncoimmunology. 2021;10(1):1880687.
Karagiannis P, Gilbert AE, Josephs DH, Ali N, Dodev T, Saul L, et al. IgG4 subclass antibodies impair antitumor immunity in melanoma. J Clin Invest. 2013;123(4):1457–74.
Lichtman A. The ABCs of ovarian cancer immunology: IgA, B cells, and CTLs. Sci Immunol. 2021;6(57):eabh3184.
Zheng J, Zhou C, Li Z, Jin X, Zou Y, Bai S, et al. Alcaligenes faecalis promotes colitis to colorectal cancer transition through IgA + B cell suppression and vinculin acetylation. Gut Microbes. 2025;17(1):2473511.
Wang R, Song S, Qin J, Yoshimura K, Peng F, Chu Y, et al. Evolution of immune and stromal cell States and ecotypes during gastric adenocarcinoma progression. Cancer Cell. 2023;41(8):1407–e269.
Park JG, Roh PR, Kang MW, Cho SW, Hwangbo S, Jung HD, et al. Intrahepatic IgA complex induces polarization of cancer-associated fibroblasts to matrix phenotypes in the tumor microenvironment of HCC. Hepatology. 2024;80(5):1074–86.
Biswas S, Mandal G, Payne KK, Anadon CM, Gatenbee CD, Chaurio RA, et al. IgA transcytosis and antigen recognition govern ovarian cancer immunity. Nature. 2021;591(7850):464–70.
Mandal G, Biswas S, Anadon CM, Yu X, Gatenbee CD, Prabhakaran S, et al. Iga-dominated humoral immune responses govern patients’ outcome in endometrial cancer. Cancer Res. 2022;82(5):859–71.
Shalapour S, Lin XJ, Bastian IN, Brain J, Burt AD, Aksenov AA, et al. Inflammation-induced IgA + cells dismantle anti-liver cancer immunity. Nature. 2017;551(7680):340–5.
Ma Y, Wang X, Qiu C, Qin J, Wang K, Sun G, et al. Using protein microarray to identify and evaluate autoantibodies to tumor-associated antigens in ovarian cancer. Cancer Sci. 2021;112(2):537–49.
Reuschenbach M, von Knebel Doeberitz M, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother. 2009;58(10):1535–44.
Banville AC, Nelson BH. Breaching B cell tolerance in the tumor microenvironment. Cancer Cell. 2022;40(4):356–8.
Campa MJ, Gottlin EB, Wiehe K, Patz EF. Jr. A tumor-binding antibody with cross-reactivity to viral antigens. Cancer Immunol Immunother. 2025;74(4):126.
Yao M, Preall J, Yeh JT, Pappin D, Cifani P, Zhao Y, et al. Plasma cells in human pancreatic ductal adenocarcinoma secrete antibodies against self-antigens. JCI Insight. 2023;8(21):e172449.
Dai Y, Aizenbud L, Qin K, Austin M, Jaycox JR, Cunningham J, et al. Humoral determinants of checkpoint immunotherapy. Nature. 2025;644(8076):527–36.
Wieland A, Patel MR, Cardenas MA, Eberhardt CS, Hudson WH, Obeng RC, et al. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature. 2021;597(7875):274–8.
Gao J, Gu D, Yang K, Zhang J, Lin Q, Yuan W, et al. Infiltrating plasma cells maintain glioblastoma stem cells through IgG-Tumor binding. Cancer Cell. 2025;43(1):122–e438.
Zhang Y, Xu M, Ren Y, Ba Y, Liu S, Zuo A, et al. Tertiary lymphoid structural heterogeneity determines tumour immunity and prospects for clinical application. Mol Cancer. 2024;23(1):75.
Schürch CM, Bhate SS, Barlow GL, Phillips DJ, Noti L, Zlobec I, et al. Coordinated cellular neighborhoods orchestrate antitumoral immunity at the colorectal cancer invasive front. Cell. 2020;182(5):1341–e5919.
Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, et al. Innate immune landscape in early lung adenocarcinoma by paired Single-Cell analyses. Cell. 2017;169(4):750–e6517.
Cui C, Wang J, Fagerberg E, Chen PM, Connolly KA, Damo M, et al. Neoantigen-driven B cell and CD4 T follicular helper cell collaboration promotes anti-tumor CD8 T cell responses. Cell. 2021;184(25):6101–e1813.
Hollern DP, Xu N, Thennavan A, Glodowski C, Garcia-Recio S, Mott KR, et al. B cells and T follicular helper cells mediate response to checkpoint inhibitors in high mutation burden mouse models of breast cancer. Cell. 2019;179(5):1191–e20621.
Xue J, Yang S, Zhang SS, Fan J, Wu ZL, Sui CJ, et al. Deciphering the multifaceted immune landscape of unresectable primary liver cancer to predict immunotherapy response. Adv Sci (Weinh). 2024;11(47):e2309631.
Hu C, You W, Kong D, Huang Y, Lu J, Zhao M, et al. Tertiary lymphoid structure-associated B cells enhance CXCL13(+)CD103(+)CD8(+) tissue-resident memory T-cell response to programmed cell death protein 1 blockade in cancer immunotherapy. Gastroenterology. 2024;166(6):1069–84.
Im SJ, Obeng RC, Nasti TH, McManus D, Kamphorst AO, Gunisetty S, et al. Characteristics and anatomic location of PD-1(+)TCF1(+) stem-like CD8 T cells in chronic viral infection and cancer. Proc Natl Acad Sci U S A. 2023;120(41):e2221985120.
Siddiqui I, Schaeuble K, Chennupati V, Fuertes Marraco SA, Calderon-Copete S, Pais Ferreira D, et al. Intratumoral Tcf1(+)PD-1(+)CD8(+) T cells with Stem-like properties promote tumor control in response to vaccination and checkpoint Blockade immunotherapy. Immunity. 2019;50(1):195–e21110.
Kurtulus S, Madi A, Escobar G, Klapholz M, Nyman J, Christian E, et al. Checkpoint Blockade immunotherapy induces dynamic changes in PD-1(-)CD8(+) Tumor-Infiltrating T cells. Immunity. 2019;50(1):181–. – 94.e6.
Lee J, Lee K, Bae H, Lee K, Lee S, Ma J, et al. IL-15 promotes self-renewal of progenitor exhausted CD8 T cells during persistent antigenic stimulation. Front Immunol. 2023;14:1117092.
Kim KH, Kim HK, Kim HD, Kim CG, Lee H, Han JW, et al. PD-1 blockade-unresponsive human tumor-infiltrating CD8(+) T cells are marked by loss of CD28 expression and rescued by IL-15. Cell Mol Immunol. 2021;18(2):385–97.
Steiner C, Denlinger N, Huang X, Yang Y. Stem-like CD8(+) T cells in cancer. Front Immunol. 2024;15:1426418.
Ni L. Potential mechanisms of cancer stem-like progenitor T-cell bio-behaviours. Clin Transl Med. 2024;14(8):e1817.
Zander R, Kasmani MY, Chen Y, Topchyan P, Shen J, Zheng S, et al. Tfh-cell-derived Interleukin 21 sustains effector CD8(+) T cell responses during chronic viral infection. Immunity. 2022;55(3):475–e935.
Krueger PD, Osum KC, Jenkins MK. CD4(+) memory T-cell formation during type 1 immune responses. Cold Spring Harb Perspect Biol. 2021;13(12):a038141.
Magen A, Hamon P, Fiaschi N, Soong BY, Park MD, Mattiuz R, et al. Intratumoral dendritic cell-CD4(+) T helper cell niches enable CD8(+) T cell differentiation following PD-1 blockade in hepatocellular carcinoma. Nat Med. 2023;29(6):1389–99.
Cappuyns S, Philips G, Vandecaveye V, Boeckx B, Schepers R, Van Brussel T, et al. PD-1(-) CD45RA(+) effector-memory CD8 T cells and CXCL10(+) macrophages are associated with response to Atezolizumab plus bevacizumab in advanced hepatocellular carcinoma. Nat Commun. 2023;14(1):7825.
Ramachandran M, Vaccaro A, van de Walle T, Georganaki M, Lugano R, Vemuri K, et al. Tailoring vascular phenotype through AAV therapy promotes anti-tumor immunity in glioma. Cancer Cell. 2023;41(6):1134–e5110.
Tanoue K, Ohmura H, Uehara K, Ito M, Yamaguchi K, Tsuchihashi K, et al. Spatial dynamics of CD39(+)CD8(+) exhausted T cell reveal tertiary lymphoid structures-mediated response to PD-1 blockade in esophageal cancer. Nat Commun. 2024;15(1):9033.
Sadeghirad H, Monkman J, Tan CW, Liu N, Yunis J, Donovan ML, et al. Spatial dynamics of tertiary lymphoid aggregates in head and neck cancer: insights into immunotherapy response. J Transl Med. 2024;22(1):677.
Wolf Y, Anderson AC, Kuchroo VK. TIM3 comes of age as an inhibitory receptor. Nat Rev Immunol. 2020;20(3):173–85.
Ando M, Ito M, Srirat T, Kondo T, Yoshimura A. Memory T cell, exhaustion, and tumor immunity. Immunol Med. 2020;43(1):1–9.
Zhang D, Jiang D, Jiang L, Ma J, Wang X, Xu X, et al. HLA-A(+) tertiary lymphoid structures with reactivated tumor infiltrating lymphocytes are associated with a positive immunotherapy response in esophageal squamous cell carcinoma. Br J Cancer. 2024;131(1):184–95.
Jansen CS, Prokhnevska N, Master VA, Sanda MG, Carlisle JW, Bilen MA, et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature. 2019;576(7787):465–70.
Yao Z, Li G, Pan D, Pei Z, Fang Y, Liu H, et al. Roles and functions of tumor-infiltrating lymphocytes and tertiary lymphoid structures in gastric cancer progression. Front Immunol. 2025;16:1595070.
Cai D, Yu H, Wang X, Mao Y, Liang M, Lu X, et al. Turning tertiary lymphoid structures (TLS) into hot spots: values of TLS in gastrointestinal tumors. Cancers (Basel). 2023;15(2):367.
Zheng S, Wang W, Shen L, Yao Y, Xia W, Ni C. Tumor battlefield within inflamed, excluded or desert immune phenotypes: the mechanisms and strategies. Exp Hematol Oncol. 2024;13(1):80.
Italiano A, Bessede A, Pulido M, Bompas E, Piperno-Neumann S, Chevreau C, et al. Pembrolizumab in soft-tissue sarcomas with tertiary lymphoid structures: a phase 2 PEMBROSARC trial cohort. Nat Med. 2022;28(6):1199–206.
Vanhersecke L, Brunet M, Guégan JP, Rey C, Bougouin A, Cousin S, et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat Cancer. 2021;2(8):794–802.
Ruffin AT, Cillo AR, Tabib T, Liu A, Onkar S, Kunning SR, et al. B cell signatures and tertiary lymphoid structures contribute to outcome in head and neck squamous cell carcinoma. Nat Commun. 2021;12(1):3349.
Zhi Y, Wang Q, Zi M, Zhang S, Ge J, Liu K, et al. Spatial transcriptomic and metabolomic landscapes of oral submucous fibrosis-derived oral squamous cell carcinoma and its tumor microenvironment. Adv Sci. 2024;11(12):e2306515.
Petitprez F, de Reyniès A, Keung EZ, Chen TW, Sun CM, Calderaro J, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577(7791):556–60.
Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014;189(7):832–44.
Gorvel L, Panouillot M, Rouvière MS, Billon E, Fattori S, Sonongbua J, et al. Tertiary lymphoid structures are associated with enhanced macrophage activation and immune checkpoint expression and predict outcome in cervical cancer. Cancer Immunol Res. 2025;13(5):712–28.
Kroeger DR, Milne K, Nelson BH. Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T-cell responses, and superior prognosis in ovarian cancer. Clin Cancer Res. 2016;22(12):3005–15.
Lehmann J, Thelen M, Kreer C, Schran S, Garcia-Marquez MA, Cisic I, et al. Tertiary lymphoid structures in pancreatic cancer are structurally homologous, share gene expression patterns and B-cell clones with secondary lymphoid organs, but show increased T-cell activation. Cancer Immunol Res. 2025;13(3):323–36.
Groeneveld CS, Fontugne J, Cabel L, Bernard-Pierrot I, Radvanyi F, Allory Y, et al. Tertiary lymphoid structures marker CXCL13 is associated with better survival for patients with advanced-stage bladder cancer treated with immunotherapy. Eur J Cancer. 2021;148:181–9.
Coppola D, Nebozhyn M, Khalil F, Dai H, Yeatman T, Loboda A, et al. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am J Pathol. 2011;179(1):37–45.
Shang T, Jiang T, Lu T, Wang H, Cui X, Pan Y, et al. Tertiary lymphoid structures predict the prognosis and immunotherapy response of cholangiocarcinoma. Front Immunol. 2023;14:1166497.
Wang X, Venet D, Lifrange F, Larsimont D, Rediti M, Stenbeck L, et al. Spatial transcriptomics reveals substantial heterogeneity in triple-negative breast cancer with potential clinical implications. Nat Commun. 2024;15(1):10232.
Zhang Y, Yu B, Ming W, Zhou X, Wang J, Chen D, SpaTopic. A statistical learning framework for exploring tumor Spatial architecture from Spatially resolved transcriptomic data. Sci Adv. 2024;10(39):eadp4942.
Lin J, Jiang S, Chen B, Du Y, Qin C, Song Y, et al. Tertiary lymphoid structures are linked to enhanced antitumor immunity and better prognosis in Muscle-Invasive bladder cancer. Adv Sci (Weinh). 2025;12(7):e2410998.
Lei JX, Wang R, Hu C, Lou X, Lv MY, Li C, et al. Deciphering tertiary lymphoid structure heterogeneity reveals prognostic signature and therapeutic potentials for colorectal cancer: a multicenter retrospective cohort study. Int J Surg. 2024;110(9):5627–40.
Finkin S, Yuan D, Stein I, Taniguchi K, Weber A, Unger K, et al. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat Immunol. 2015;16(12):1235–44.
Long S, Li M, Chen J, Zhong L, Dai G, Pan D, et al. Transfer learning radiomic model predicts intratumoral tertiary lymphoid structures in hepatocellular carcinoma: a multicenter study. J Immunother Cancer. 2025;13(3):e011126.
Long S, Li M, Chen J, Zhong L, Abudulimu A, Zhou L, et al. Spatial patterns and MRI-based radiomic prediction of high peritumoral tertiary lymphoid structure density in hepatocellular carcinoma: a multicenter study. J Immunother Cancer. 2024;12(12):e009879.
Yu Y, Yang T, Ma P, Zeng Y, Dai Y, Fu Y, et al. Determining the status of tertiary lymphoid structures in invasive pulmonary adenocarcinoma based on chest CT radiomic features. Insights Imaging. 2025;16(1):28.
Li Z, Jiang Y, Li B, Han Z, Shen J, Xia Y, et al. Development and validation of a machine learning model for detection and classification of tertiary lymphoid structures in gastrointestinal cancers. JAMA Netw Open. 2023;6(1):e2252553.
Mai H, Luo J, Hoeher L, Al-Maskari R, Horvath I, Chen Y, et al. Whole-body cellular mapping in mouse using standard IgG antibodies. Nat Biotechnol. 2024;42(4):617–27.
Leiter RE. The power of hope. Cell. 2023;186(8):1518–22.
Jammihal T, Saliby RM, Labaki C, Soulati H, Gallegos J, Peris A, et al. Immunogenomic determinants of exceptional response to immune checkpoint inhibition in renal cell carcinoma. Nat Cancer. 2025;6(2):372–84.
Chung SY, Yeh YC, Huang CJ, Chiang NJ, Hsu DS, Chan MH, et al. Comparative impact of tertiary lymphoid structures and tumor-infiltrating lymphocytes in cholangiocarcinoma. J Immunother Cancer. 2025;13(1):e010173.
Levy A, Morel D, Texier M, Rodriguez-Ruiz ME, Bouarroudj L, Bouquet F, et al. Monocyte-lineage tumor infiltration predicts immunoradiotherapy response in advanced pretreated soft-tissue sarcoma: phase 2 trial results. Signal Transduct Target Ther. 2025;10(1):103.
Li J, Zhang L, Xing H, Geng Y, Lv S, Luo X, et al. The absence of intra-tumoral tertiary lymphoid structures is associated with a worse prognosis and mTOR signaling activation in hepatocellular carcinoma with liver transplantation: a multicenter retrospective study. Adv Sci. 2024;11(21):e2309348.
Peyraud F, Guégan JP, Rey C, Lara O, Odin O, Del Castillo M, et al. Spatially resolved transcriptomics reveal the determinants of primary resistance to immunotherapy in NSCLC with mature tertiary lymphoid structures. Cell Rep Med. 2025;6(2):101934.
Elfving H, Yu H, Fessehatsion KK, Brunnström H, Botling J, Gulyas M, et al. Spatial distribution of tertiary lymphoid structures in the molecular and clinical context of non-small cell lung cancer. Cell Oncol (Dordr). 2025;48(3):801–13.
Wang YQ, Chen WJ, Zhou W, Dong KQ, Zuo L, Xu D, et al. Integrated analysis of tertiary lymphoid structures and immune infiltration in CcRCC microenvironment revealed their clinical significances: a multicenter cohort study. J Immunother Cancer. 2024;12(6):e008613.
Hugaboom MB, Wirth LV, Street K, Ruthen N, Jegede OA, Schindler NR, et al. Presence of tertiary lymphoid structures and exhausted tissue-resident T cells determines clinical response to PD-1 blockade in renal cell carcinoma. Cancer Discov. 2025;15(5):948–68.
Wang J, Liang Y, Xue A, Xiao J, Zhao X, Cao S, et al. Intratumoral CXCL13(+) CD160(+) CD8(+) T cells promote the formation of tertiary lymphoid structures to enhance the efficacy of immunotherapy in advanced gastric cancer. J Immunother Cancer. 2024;12(9):e009603.
Zhang X, Li L, Shi X, Zhao Y, Cai Z, Ni N, et al. Development of a tertiary lymphoid structure-based prognostic model for breast cancer: integrating single-cell sequencing and machine learning to enhance patient outcomes. Front Immunol. 2025;16:1534928.
Zhao YY, Fan Z, Tao BR, Du ZG, Shi ZF. Density of tertiary lymphoid structures predicts clinical outcome in breast cancer brain metastasis. J Immunother Cancer. 2024;12(7):e009232.
Wu C, Zhang G, Wang L, Hu J, Ju Z, Tao H, et al. Spatial proteomic profiling elucidates immune determinants of neoadjuvant chemo-immunotherapy in esophageal squamous cell carcinoma. Oncogene. 2024;43(37):2751–67.
Lu H, Lou H, Wengert G, Paudel R, Patel N, Desai S, et al. Tumor and local lymphoid tissue interaction determines prognosis in high-grade serous ovarian cancer. Cell Rep Med. 2023;4(7):101092.
Li C, Wang P, Dong Z, Cao W, Su Y, Zhang J, et al. Single-cell transcriptomics analysis reveals that the tumor-infiltrating B cells determine the indolent fate of papillary thyroid carcinoma. J Exp Clin Cancer Res. 2025;44(1):91.
Chang TG, Spathis A, Schäffer AA, Gavrielatou N, Kuo F, Jia D, et al. Tumor and blood B-cell abundance outperforms established immune checkpoint blockade response prediction signatures in head and neck cancer. Ann Oncol. 2025;36(3):309–20.
Gavrielatou N, Fortis E, Spathis A, Anastasiou M, Economopoulou P, Foukas GRP, et al. B-cell infiltration is associated with survival outcomes following programmed cell death protein 1 inhibition in head and neck squamous cell carcinoma. Ann Oncol. 2024;35(4):340–50.
Westbom-Fremer S, Tran L, Ebbesson A, Martin de la Fuente L, Jönsson JM, Kannisto P, et al. Tertiary lymphoid structures in high-grade serous tubo-ovarian carcinoma: anatomical site matters. Cancer Immunol Immunother. 2025;74(2):56.
Zhang R, Jin T, Ren Y, Wu SM, Wu Y, Liu XJ, et al. Harnessing nanotheranostics-based dendritic cells tracking mature tertiary lymphoid structures to boost anti-Glioma immunotherapy. Adv Funct Mater. 2025:2425894.
Kuang G, Zhang Q, Li W, Zhao Y. Biomimetic tertiary lymphoid structures with microporous annealed particle scaffolds for cancer postoperative therapy. ACS Nano. 2024;18(12):9176–86.
Ahn W, Han J, Kim N, Hwang YH, Kim W, Lee Y, et al. Hierarchical protein nano-crystalline hydrogel with extracellular vesicles for ectopic lymphoid structure formation. Biomaterials. 2025;318:123166.
Zhang L, Zhang B, Zhang MJ, Li W, Li H, Jiao Y, et al. Trigger inducible tertiary lymphoid structure formation using covalent organic frameworks for cancer immunotherapy. Nat Commun. 2025;16(1):44.
Yang QC, Wang YY, Wang S, Song A, Wang WD, Zhang L, et al. Engineered bacterial membrane biomimetic covalent organic framework as nano-immunopotentiator for cancer immunotherapy. Bioact Mater. 2025;47:283–94.
Hu L, Li T, Deng S, Gao H, Jiang Y, Chen Q, et al. Tertiary lymphoid structure formation induced by LIGHT-engineered and photosensitive nanoparticles-decorated bacteria enhances immune response against colorectal cancer. Biomaterials. 2025;314:122846.
Cheng R, Li S, Ma X, Zhuang W, Lei Y, He J, et al. Intratumoral antigen-presenting cell activation by a nanovesicle for the concurrent tertiary lymphoid structure de novo neogenesis. Sci Adv. 2025;11(8):eadr1299.
Jin XK, Liang JL, Zhang SM, Ji P, Huang QX, Qin YT, et al. Engineering metal-based hydrogel-mediated tertiary lymphoid structure formation via activation of the STING pathway for enhanced immunotherapy. Mater Horiz. 2023;10(10):4365–79.
Li M, Lu L, Bao Q, Zhou M, Nie B, Liu Y, et al. Radiotherapy-derived engineered stem cell exosomes improve anti-glioma immunotherapy by promoting the formation of tertiary lymphoid structure and improve the release of type I interferon. J Nanobiotechnol. 2025;23(1):239.
Zhang L, Zhang R, Jin D, Zhang T, Shahatiaili A, Zang J, et al. Synergistic induction of tertiary lymphoid structures by chemoimmunotherapy in bladder cancer. Br J Cancer. 2024;130(7):1221–31.
Wang L, Wan L, Chen X, Gao P, Hou Y, Wu L, et al. Reduced intestinal-to-diffuse conversion and immunosuppressive responses underlie superiority of neoadjuvant immunochemotherapy in gastric adenocarcinoma. MedComm. 2024;5(11):e762.
Cascone T, Leung CH, Weissferdt A, Pataer A, Carter BW, Godoy MCB, et al. Neoadjuvant chemotherapy plus nivolumab with or without ipilimumab in operable non-small cell lung cancer: the phase 2 platform NEOSTAR trial. Nat Med. 2023;29(3):593–604.
Lanickova T, Hensler M, Kasikova L, Vosahlikova S, Angelidou A, Pasulka J, et al. Chemotherapy drives tertiary lymphoid structures that correlate with ICI-responsive TCF1 + CD8 + T cells in metastatic ovarian cancer. Clin Cancer Res. 2025;31(1):164–80.
Lu Y, Zhao Q, Liao JY, Song E, Xia Q, Pan J, et al. Complement signals determine opposite effects of B cells in Chemotherapy-Induced immunity. Cell. 2020;180(6):1081–e9724.
Lv J, Wei Y, Yin JH, Chen YP, Zhou GQ, Wei C, et al. The tumor immune microenvironment of nasopharyngeal carcinoma after gemcitabine plus cisplatin treatment. Nat Med. 2023;29(6):1424–36.
Wang D, Huang L, Qian D, Cao Y, Wu X, Xu P, et al. Low-dose radiotherapy promotes the formation of tertiary lymphoid structures in lung adenocarcinoma. Front Immunol. 2023;14:1334408.
Zhang MJ, Lin WP, Wang Q, Wang S, Song A, Wang YY, et al. Oncolytic herpes simplex virus propagates tertiary lymphoid structure formation via CXCL10/CXCR3 to boost antitumor immunity. Cell Prolif. 2025;58(1):e13740.
Li R, Villa NY, Yu X, Johnson JO, Borjas G, Dhillon J, et al. Oncolytic immunotherapy with nivolumab in muscle-invasive bladder cancer: a phase 1b trial. Nat Med. 2025;31(1):176–88.
Liu J, Wang X, Li Z, Gao S, Mao L, Dai J, et al. Neoadjuvant oncolytic virus orienx010 and Toripalimab in resectable acral melanoma: a phase Ib trial. Signal Transduct Target Ther. 2024;9(1):318.