Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Author affiliation: Linköping University, Linköping, Sweden (E. Hero, P. Forsberg, P.-E. Lindgren, A.J. Henningsson, P. Wilhelmsson); Region Jönköping County, Jönköping, Sweden (M. Lager, P.-E. Lindgren, A.J. Henningsson, P. Wilhelmsson)
Neoehrlichia mikurensis is an emerging tickborne pathogen, mainly transmitted by Ixodes ricinus ticks. The human infection known as neoehrlichiosis was first reported in Sweden in 2010 and has been documented in several ountries in Europe (1,2). Neoehrlichiosis affects both healthy and immunocompromised persons, and more severe symptoms, such as vascular complications, are typically seen in immunocompromised patients (3). Diagnosis is challenging because of vague symptoms, low awareness, and lack of commercial diagnostic tests. PCR is the primary method of detection, because N. mikurensis is difficult to culture and no serologic tests are available. The true prevalence of N. mikurensis in humans and ticks remains unclear, although its DNA has been detected in both throughout Europe (4,5).
We investigated the prevalence of N. mikurensis after tick bites in northern Europe, aiming to detect its DNA in ticks and human blood and to assess symptoms among participants. The samples were originally collected in 2008–2009, when N. mikurensis was not yet recognized as a human pathogen and appropriate diagnostic tools were lacking; therefore, this analysis could not be conducted until recently. Despite being >15 years old, the data offer valuable insight into N. mikurensis ecology and transmission. Although epidemiology might have shifted, the underlying biologic mechanisms of pathogen transmission likely remain unchanged, ensuring continued relevance.
Figure
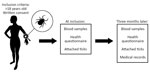
Figure. Schematic overview of the Tick-Borne Diseases STING study during 2008–2009 in Sweden and Finland (6), in which tick-bitten persons >18 years of age gave written informed…
We analyzed data from the Tick-Borne Diseases (TBD) STING study (6,7), a prospective multicenter study conducted in Sweden and on the Åland Islands, Finland (Figure). During May 2008–November 2009, primary healthcare centers enrolled 1,425 healthy tick-bitten adults (>18 years of age; median age 63, range 19–92) through public advertisements. The study was approved in 2008 by the Regional Ethical Review Board in Linköping, Sweden, and the Åland Health Care Ethics Committee.
At study inclusion, participants submitted removed tick(s), provided a blood sample, and completed a questionnaire on tick exposure, prior tickborne diseases, current health status, and medication use (8). Participants on antimicrobial drugs or immunosuppressive therapy were excluded. A follow-up visit 3 months later included providing a second blood sample and filling out a questionnaire about symptoms experienced since inclusion and if the participant had sought medical care (8). Medical records were reviewed when relevant. Participants also submitted any additional ticks found attached during the 3-month study period.
For this study, we photographed each tick and identified it by species, developmental stage, and sex. We estimated feeding duration for nymphs and adult females (9). We homogenized ticks, extracted total nucleic acids, and reverse-transcribed to cDNA (7). Participant plasma was collected in EDTA tubes (6), and we extracted DNA from participants whose ticks were PCR-positive for N. mikurensis.
We screened tick cDNA for N. mikurensis by using a SYBR Green real-time PCR targeting the 16S rRNA gene (10) and confirming positive results by using a TaqMan real-time PCR targeting the groEL gene (11). We analyzed plasma DNA from participants bitten by positive ticks by using the same TaqMan assay. We confirmed PCR products by sequencing (Macrogen Inc., https://www.macrogen.com) and verified sequences with BLAST (https://blast.ncbi.nlm.nih.gov). We used a previously confirmed N. mikurensis-positive cDNA sample as a positive PCR control (10).
Of the 1,644 I. ricinus ticks analyzed, 18 (1.1%) were positive for N. mikurensis in both PCRs (Table 1). No DNA was found in larvae. Prevalence in nymphs was 1.1% and in adult females was 1.3%. The ticks were collected from participants in various regions in Sweden and on the Åland Islands (Appendix Table). Of the 18 participants bitten by PCR-positive ticks, 2 (11%) tested positive for N. mikurensis (Table 2).
The first positive participant was a 41-year-old otherwise healthy woman from southern Sweden who tested positive at inclusion. She had been bitten by a N. mikurensis–positive nymph that had fed for
The second positive participant was a 68-year-old otherwise healthy woman from south-central Sweden, who tested positive at the 3-month follow-up. She had been bitten by a N. mikurensis–positive nymph that had fed for ≈40 hours. She reported fatigue, neck pain, myalgia, and numbness but did not seek medical care. She was bitten by 3 additional ticks during the study period, of which only 1 was submitted to the TBD STING study; it tested negative for N. mikurensis.
Sequencing confirmed N. mikurensis DNA in both the ticks and the blood samples from the 2 participants. The remaining 16 participants bitten by PCR-positive ticks tested PCR-negative for N. mikurensis in both the inclusion and follow-up blood samples. Out of those participants, 9 reported no symptoms during the study period and 2 did not respond to the follow-up questionnaire. The remaining 5 reported nonspecific symptoms, including muscle and joint pain (n = 5), headache (n = 2), fatigue (n = 2), neck pain (n = 1), vertigo (n = 2), and numbness (n = 2).
We detected N. mikurensis DNA in 1.1% of I. ricinus ticks that had bitten humans in Sweden and Finland, indicating a low prevalence in the study areas compared with those reported in other regions of Sweden (10,12). Two participants bitten by PCR-positive ticks also tested positive for N. mikurensis in blood samples and reported symptoms consistent with neoehrlichiosis, despite being immunocompetent. Those findings suggest that, although the overall risk for infection after a tick bite is low, transmission can still occur even after short tick attachment times (
The prospective design of the TBD STING study, large number of samples, and standardized follow-up strengthen the reliability of our findings. The use of a 2-tier PCR targeting the 16S rRNA and groEL genes and then sequencing ensured high specificity of the detection assay. By linking infected ticks to the person they had bitten, the study provided an opportunity to assess the risk for transmission under natural conditions.
Although PCR detects bacterial DNA rather than viable organism, its sensitivity depends on having enough N. mikurensis DNA present in the blood sample. Because N. mikurensis likely causes low-level bacteremia because of its tissue tropism (13,14), the pathogen might not always be detectable in blood samples, leading to false-negative results and potential underdiagnosis. Therefore, the symptoms reported by PCR-negative participants bitten by positive ticks might be related to N. mikurensis exposure. Furthermore, PCR cannot detect seroconversion or immune responses, and the absence of serologic tools limits the ability of this study to identify resolved infections.
The absence of N. mikurensis DNA in tick larvae supports evidence that transovarial transmission is unlikely (10,12,15), whereas similar prevalence in nymphs and adult females suggests stable infection rates across stages, informing our understanding of pathogen maintenance in tick populations. The relevance of our results likely extends beyond the specific regions studied. Therefore, in areas where N. mikurensis has been detected in ticks, clinicians should consider this pathogen in the differential diagnosis of patients with unexplained symptoms after a tick bite.
Dr. Hero is currently doing her residency in internal medicine at the Hospital of Kiruna. Her research interests include ticks and tickborne infections.
We thank the study participants and the staff at the primary healthcare centers involved in the Tick-Borne Diseases STING study. We thank the Tick-Borne Diseases STING study group for their contributions to the study design and logistical planning. We thank Anna Angel, Marko Lindsjö, and Thomas Rönnmark for their laboratory assistance.
This study was supported by The Swedish Research Council (Branch of Medicine grant no. K2008-58X-14631-06-3); The Medical Research Council of Southeast Sweden (grant no. 931010); the Division of Laboratory Medicine, Region Jönköping County; and the European Regional Development Fund and the Interreg NorthSea Region Programme 2014–2020 as part of the NorthTick project (reference no. J-No: 38-2-7-19).
P.E.L. is an external, scientific advisor to Bavarian-Nordic A/S and Pfizer Inc. P.E.L. and A.H. have a research collaboration agreement with Pfizer. The other authors report no conflicts of interest.
