A team of researchers has demonstrated that well-decomposed peat could serve as a sustainable and highly efficient precursor for next-gen fuel cell catalysts and potentially replace expensive platinum.
The study was carried out by scientists from the Helmholtz-Zentrum Berlin (HZB), the Physikalisch-Technische Bundesanstalt (PTB) and the universities of Tartu and Tallinn in Estonia.
They aimed to create a platinum-free catalyst for the oxygen reduction reaction in anion exchange membrane fuel cells, where platinum remains the dominant cost driver. Iron–nitrogen–carbon (Fe-N-C) catalysts could offer a cheaper alternative to today’s platinum catalysts currently used in fuel cells.
To observe the formation of complex microstructures within various samples of the iron-nitrogen-carbon (Fe-N-C) catalysts, the scientists used advanced X-ray scattering at the third-generation BESSY II synchrotron radiation source.
Fuel cell efficiency boost
Fuel cells convert hydrogen into electricity with water as the only by-product. But even though the technology is fundamental role for long-term climate-neutral energy strategies, platinum still remains a massive obstacle.
The silvery white precious metal is currently the standard catalyst for the oxygen reduction reaction (ORR), the slow step that determines how efficiently fuel cells operate. Replacing it with cheaper, abundant materials could greatly cut fuel cell production costs.
 Efficiency and selectivity of the reactions in an Anion Exchange Membrane Fuel Cell can be increased by the complex pore structure of the Fe-N-C-electrocatalyst.
Efficiency and selectivity of the reactions in an Anion Exchange Membrane Fuel Cell can be increased by the complex pore structure of the Fe-N-C-electrocatalyst.
Credit: R. Jäger / University Tartu
Meanwhile, carbon-based catalysts containing iron and nitrogen are a promising option for this purpose and can be used in anion exchange membrane fuel cells.
Some carbon-based materials are highly porous, with interconnected pores that function like an ant colony. Hydrogen and oxygen atoms move through these passages to reach the active catalytic sites. The resulting water is carried out the same way.
“By changing the hierarchical structure of the catalyst, the size and thickness of the pore walls, we can produce materials with very different properties,” Rutha Jäger, PhD, an associate professor at the University of Tartu and first author of the study, said.
Peat as catalyst precursor
Eneli Härk, an electrochemist at HZB, revealed the team wanted to understand why some peat-derived samples performed exceptionally while others lagged. To investigate this, they examined the materials at BESSY II using small-angle X-ray scattering (SAXS) and anomalous SAXS.
The synchrotron’s high-precision beam allowed them to quantify structural disorder, porosity, pore curvature and the distribution of iron-containing active centers. Härk stated that such parameters are nearly impossible to capture with standard methods.
The scientists synthesized five catalyst samples at different temperatures. These ranged from 1,472 to 1,832 degrees Fahrenheit (800 to 1000 degrees Celsius). They also used various pore-modifying agents during production.
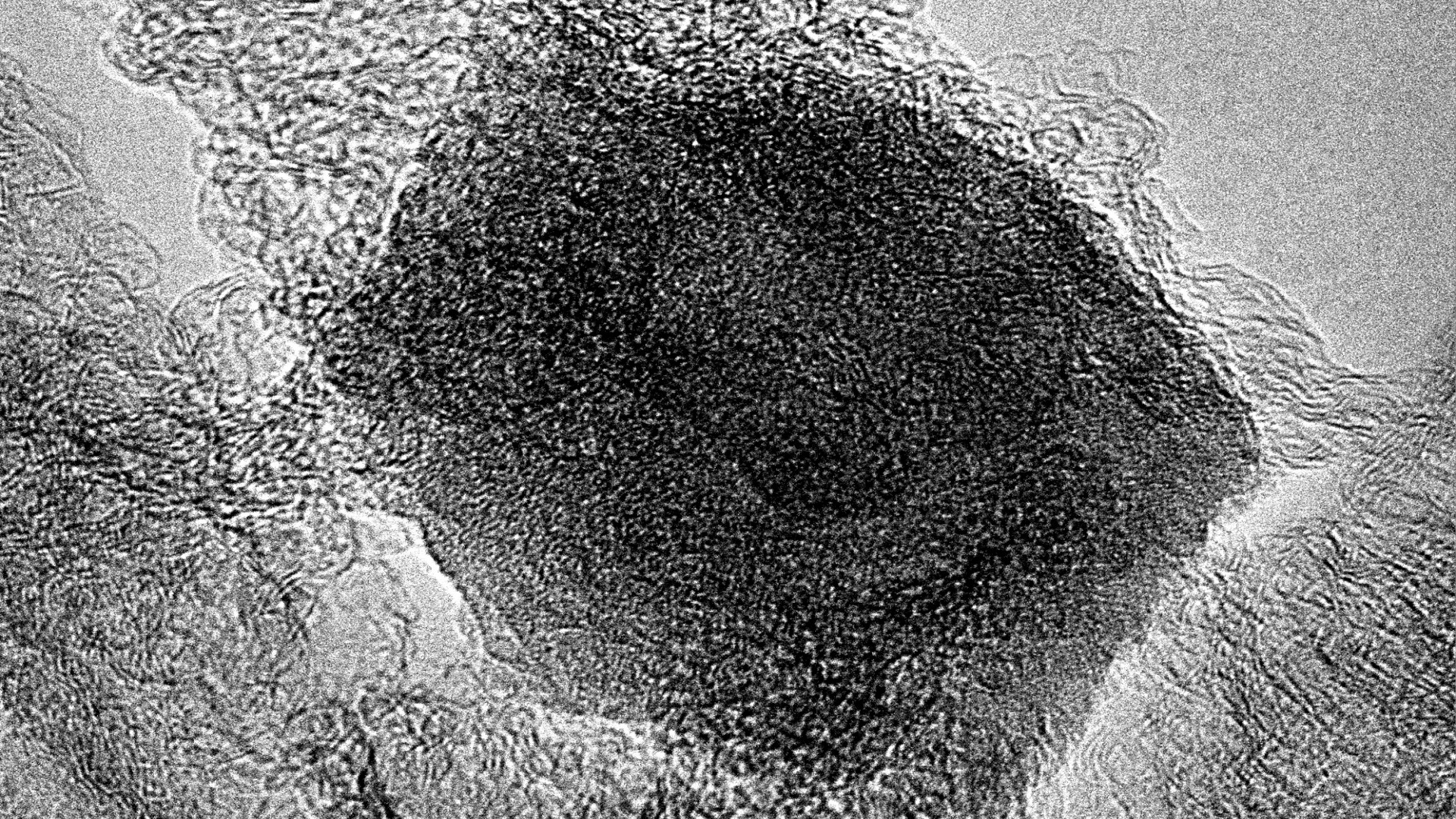 The complex and intricate structure inside the Fe-N-Carbon material.
The complex and intricate structure inside the Fe-N-Carbon material.
Credit: HZB
The X-ray data revealed 13 structural factors that influence catalytic efficiency. The team found that a pore curvature of at least three nanometers enables more efficient oxygen reduction. It also limits unwanted hydrogen peroxide formation.
“Small-angle scattering provides us with a precise map of the anthill, so to speak, while the electrochemical behaviour of the catalyst shows us how the ‘ants’, i.e. the molecules, move within it,” Härk said.
The findings showed why one peat-derived Fe-N-C catalyst performed as well as some top non-noble metal catalysts. Improving pore structure, rather than only the composition, is crucial for designing next-generation electrocatalysts.
“Estonian deposits offer a promising resource for producing high-tech functional materials,” Jäger concluded in a press release. She believes that peat can support the production of fuel cells with lower costs and greater sustainability.
The study has been published in the journal ACS Nano.