This retrospective study examined a cohort of patients with mBC under routine clinical practice using an electronic health record-derived deidentified database. The prevalence of BM at first mBC diagnosis was 3.4% for the overall population. In both the HER2+ and HER2− populations, the prevalence of BM appeared to increase by LOT. However, the effect was more pronounced in the HER2+ cohort, ranging from 12.5% at mBC diagnosis (any point prior to 30 days after diagnosis) and 11.2% at the start of 1 L to 33.0% at the start of 3 L. The prevalence of BM was lower among patients with HER2− mBC, ranging from 1.7% at the time of mBC diagnosis and 1.6% at the start of 1 L to 2.8% at the start of 3 L. The apparent increase in prevalence of BM by LOT could be a result of the ‘survival effect’, whereby patients who progress onto later lines of treatment are likely to have been living with cancer for longer than those who did not, and therefore, have spent more time at risk of developing brain metastases. Further, it is possible that patients who progress onto subsequent lines of therapy are more likely to be screened for BM, or otherwise have their BM detected, than those earlier in their disease course. However, there may also be other factors which could contribute to this observation.
In a recent study using the same database, including patients diagnosed with HER2+ mBC from January 2016 to May 2019, DeBusk et al. found a prevalence of BM at mBC diagnosis of 9.9% [7]. However, this study included BM present at the mBC diagnosis date. In contrast, the current study considered patients as having BM at mBC diagnosis if their date of BM was within 30 days after their diagnosis date to account for gaps in data capture. A database study of US community oncology practices which included adult, female patients diagnosed with mBC between 2012 and 2018 found 22% of patients to have BM at the time of mBC diagnosis, with a 39% prevalence during 1 L treatment and a 14% prevalence during 2 L treatment. The difference in prevalences found by this study compared with other reports and results reported here may be because the population included only patients who received at least two lines of systemic therapy, and therefore may not be representative of the overall HER2+ population. It should also be noted that the Vidal study included only 372 patients, compared with 1923 patients with HER2+ mBC in this study [21]. Other national studies have reported that 12–15% of patients with HER2+ mBC have BM at the time of mBC diagnosis [18, 19]. Prevalence data for the overall HER2− population have not been widely reported, but the prevalences for HR+, HER2− and HR−, HER2− subgroups in the current study are considerably lower than were reported in a study using the Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute from 2010 to 2013, which included patients with HR+, HER2− (5.5%) and HR−, HER2− (11.4%) breast cancer [20]. Of note, the prevalence given in the SEER study is for patients with BM at diagnosis of de novo mBC, where most patients in our study had mBC that had progressed from an earlier stage. In addition to providing more recent data, our study addresses gaps in the SEER study by reporting on patients with recurrent disease and describing BM prevalence across the treatment pathway [20].
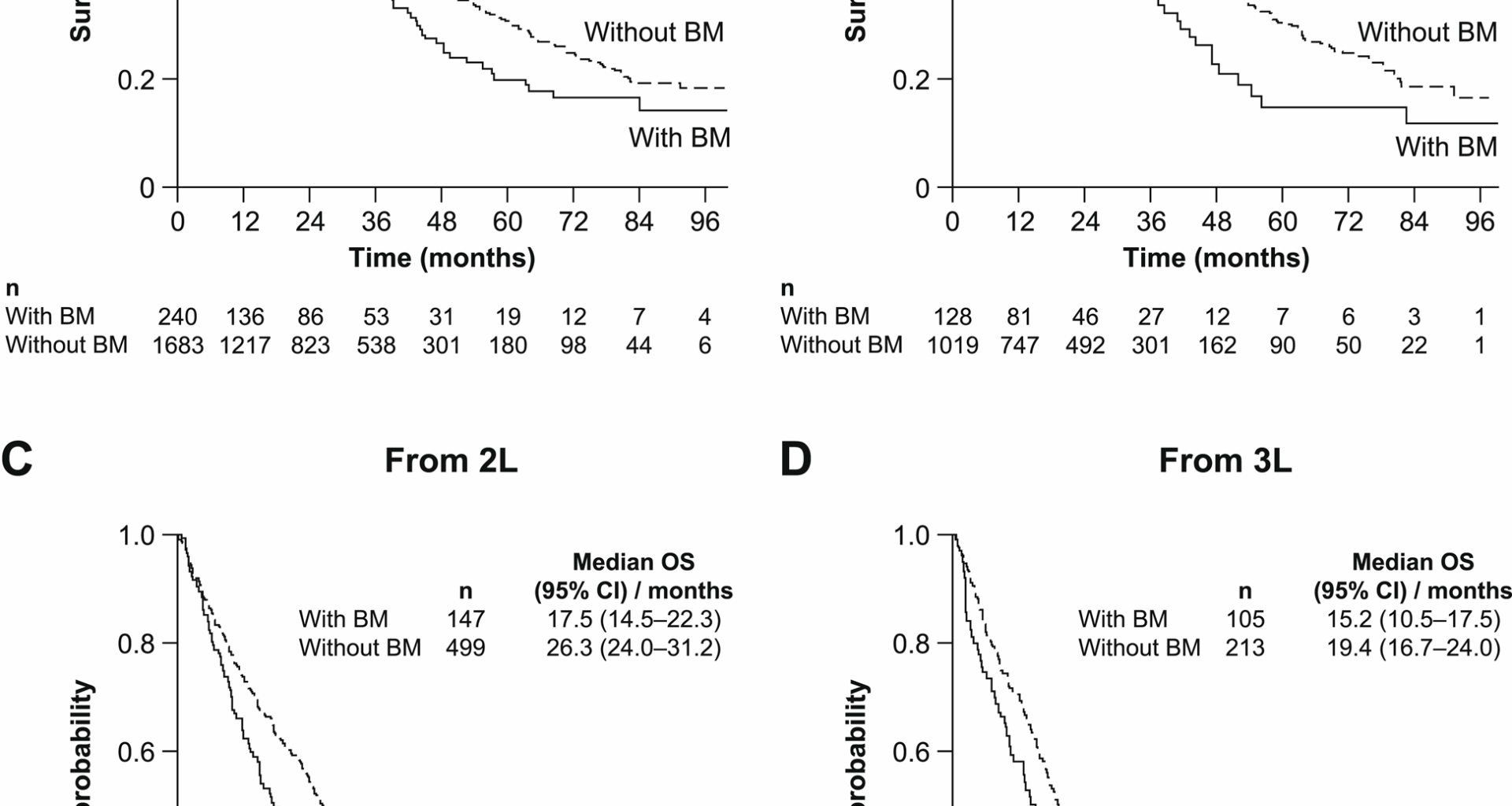
In general, survival was poorer for patients with BM than those without, and also poorer for patients with HER2− disease than for patients with HER2+ disease. Further, our study results also seem to indicate that patients with BM generally had reduced OS versus patients without BM, independent of the timing of the BM diagnosis or treatments received. Median OS was 23.9 months for patients with HER2+ mBC and BM at the diagnosis date and 36.6 months for patients without BM at mBC diagnosis. These results are similar to those from the Debusk study [7], although median OS by LOT was shorter than that reported in the Vidal study, where a median was not reached in all groups except patients with BM at diagnosis in 1 L (53.0 months) [21]. In patients with HER2− mBC, survival was also poorer in patients with BM than in those without, being 12.4 versus 27.1 months, respectively. The median OS in patients with HR+, HER2− mBC and BM is consistent with data from the SEER database (13.6 vs. 14.0 months), but was found to be longer in this study for the HR−, HER2− group (10.0 vs. 6.0 months) [20]. The poor outcomes for patients with HER2− disease as a whole highlight the unmet need in this population, despite the low overall prevalence of BM in this group.
Both TTNT and TTD were longer for patients without BM than for patients with BM in both HER2+ and HER2− mBC. The heterogeneity of the patient population, censoring of patients who did not receive a next treatment or who had no indication of discontinuation or death, and variability of treatment in real-world clinical practice compared with carefully controlled clinical trials mean that these time-to-event outcomes may not be expected to align with those expected based on the literature. The TTNT at 1 L in patients with HER2+ mBC and BM (7.9 months) was consistent with the median progression-free survival (mPFS) reported in the 2014–2020 cohort from a retrospective observational study [16]. However, the TTNT in patients with HR+, HER2− mBC (6.9 months in 1 L treatment of patients without BM) was somewhat shorter than mPFS (> 10 months) reported in clinical studies of letrozole and anastrozole in 1 L [28,29,30,31,32].
Regarding treatment patterns for patients in this analysis, three-quarters of patients with HER2+ mBC and BM at the start of 1 L were not receiving NCCN Guideline-recommended BM-specific systemic therapies. Use of these recommended therapies was higher beyond 1 L therapy, with 65.3%, 47.6%, and 45.6% receiving them as 2 L, 3 L, and 4 L + therapy, respectively. DeBusk et al. found that only 11.8% of patients with HER2+ mBC and BM were receiving NCCN Guideline-recommended treatments in 1 L, increasing to 13.0% at 2 L, 16.9% at 3 L, and then decreasing to 9% at 4 L [7]. While the low use of recommended therapies appears consistent across the US community practices that contribute data to the electronic health record-derived deidentified database, the smaller uptake of recommended systemic therapies reported in the Debusk et al. analysis may be attributed to a narrower definition of BM-recommended treatment than was used in our study [7].
In the population with HER2− mBC, the majority of patients did not receive an NCCN Guideline-recommended systemic treatment at any LOT. At 1 L, 87% did not receive a recommended therapy. The proportion receiving a recommended therapy was slightly higher in later lines, being around one-quarter in 2 L and 4 L+, and around one-third in 3 L. At all LOTs, the proportion of patients with BM receiving an NCCN Guideline-recommended therapy for BM was numerically lower for patients with HER2− disease than for HER2+. Results indicate a general prioritization of treatment to the primary tumor over BM-specific therapies, and our data suggest a more specific unmet need in the HER2− patient population. However, these results must be interpreted cautiously owing to the lack of data on radiographical or surgical treatments. Indeed, data from the SEER database between 2014 and 2016 found that among patients > 65 years of age, around half received non-stereotactic brain-directed radiation, ~15% received stereotactic brain-directed radiation, and 10–20% received surgical resection [33]. In the same study, fewer than one-fifth of patients received systemic therapy in the absence of local therapy [33]. Similarly, a US single-center cohort study reported that between 2013 and 2015, only 10% of patients received no radiation therapy and, separately, that 27% received surgery [34].
The most common regimens received among patients with HER2+ mBC were broadly aligned with the Debusk et al. and Vidal et al. analyses [7, 21], as well as with treatment guidelines in place at the time of the study (i.e. before the approval of T-DXd for patients with HER2+ mBC) that suggested use of THP in 1 L and T-DM1 in 2 L [35]. Consistent with previous reports, trastuzumab-based therapies were commonly used across all lines of therapy, which may suggest that alternative treatment options are limited [7, 21].
In the HER2− population, the most common 1 L regimens were anastrozole and letrozole, regardless of BM status. In 2 L, fulvestrant + palbociclib, capecitabine monotherapy, and fulvestrant monotherapy were most common in the overall population and in the group without BM at the start of the line. Those with BM received capecitabine monotherapy and eribulin monotherapy most often at 2 L, which is somewhat consistent with the 2021 NCCN Guidelines that suggest capecitabine monotherapy, cisplatin, or etoposide (either alone or in combination) or high-dose methotrexate for non-HER2-targeted treatment of BM [26]. As in the HER2+ population, the same treatments were used across most LOTs in the groups with and without BM (capecitabine, fulvestrant, and eribulin), which may indicate a lack of treatment options, or a lack of guidance on optimal treatment sequencing. Nonetheless, the more commonly used treatments are reflective of those recommended in general according to HR status, with aromatase inhibitors, fulvestrant, and standard chemotherapy being effective in the HR+, HER2− population, while those who are HR−, HER2− may benefit from capecitabine, eribulin, or carboplatin plus bevacizumab [36].
The large cohorts constructed in the analyses reported here allowed for reasonable precision estimates. Additionally, few other databases exist with the equivalent timeliness of data availability. The results of the study are generalizable to a wide range of outpatient oncology practice groups but may not represent all sites in the US and are subject to limitations. The most significant of these is the focus on systemic therapy and the lack of information on surgery or radiation therapy, which remain the standard of care for patients with BM [10, 11]. Further, other services could have been underreported or missing, and treatments, services, and procedures provided outside of the Flatiron Health network may not be captured.
The HER2+ results include patients who had HER2+ disease as of their mBC diagnosis and do not account for any subsequent change in HER2 status. Another limitation of the study design is that we were unable to make direct comparisons between subgroups; multivariate analyses would have been informative to control for potential confounding variables and hence make any conclusions regarding survival differences more robust. A comparison between outcomes (for example, OS) in both HER2+ and HER2− groups, and between HR+, HER2− and HR−, HER2−, would be a valuable future addition to the literature. Recent developments in the treatment and diagnosis of mBC are also unaccounted for, including the subcategorization of HER2 expression levels in patients previously classified as HER2−.
Prevalence of BM diagnosis also needs to be interpreted with caution. Without per-protocol or frequent brain scans, prevalence of BM may be underestimated by those who received and/or reported scans; it is possible that a higher incidence would be found if all patients received routine screening at baseline and prior to initiation of a new LOT. Prevalence of BM by LOT may also have been affected by variable rates of screening or BM detection at each LOT. The true prevalence cannot be determined because any patients without a known HER2 status were excluded from the overall analysis. Rates of testing for BM were not available in the data.
Overall, this study provides a comprehensive examination of the prevalence of BM, treatment patterns, and other clinical outcomes of patients with mBC, stratified by HER2 status. The results contribute information on the frequency of BM across the treatment pathway, as other literature on this is scarce.