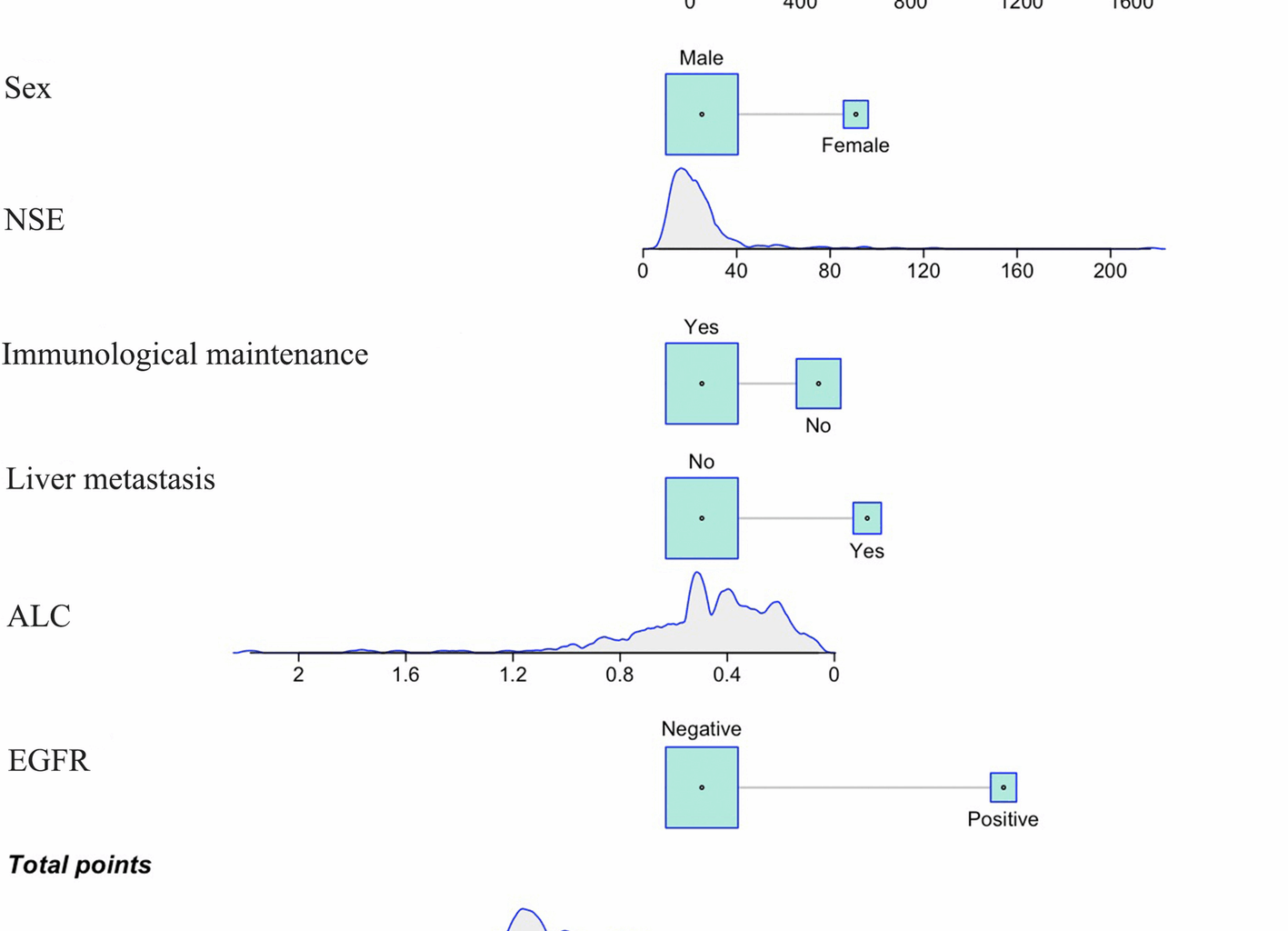
This study developed and validated a dynamic nomogram model that integrates clinicopathological features (e.g., sex, histology, EGFR status, CEA, NSE) and immune biomarkers (e.g., ALC and PD-L1 expression) for predicting the risk of BM in stage III NSCLC patients undergoing definitive CCRT. The model demonstrated favorable discrimination and calibration in both the training cohort (AUC = 0.813) and the validation cohort (AUC = 0.775). Risk stratification, with a cutoff value of 393.79, significantly differentiated survival outcomes between high-risk and low-risk patients (p = 0.034). Notably, ALC, acting as a protective factor (HR = 0.141, 95% CI: 0.015–0.941, p = 0.067), along with the survival benefit of immune maintenance therapy (p = 0.017), underscores the pivotal role of host immune status in the development of BM.
A key finding of our study was the identification of several independent predictors of BM. Understanding the biological mechanisms of these factors can help elucidate the developmental process of BM and provide a theoretical basis for clinical prevention and therapy. EGFR mutations are of significant importance in non-small cell lung cancer. Mutant EGFR can activate downstream signaling pathways, promoting tumor cell proliferation, invasion, and migration abilities [20, 21]. Additionally, tumor cells with EGFR mutations are more likely to cross the blood–brain barrier (BBB), contributing to an increased incidence of BM. This may be attributed to EGFR mutations promoting angiogenesis and increasing the permeability of the BBB, thereby facilitating tumor cell dissemination to the brain. LDH is a key enzyme in cellular glycolytic metabolism and reflects the proliferative activity and metabolic state of tumor cells [22]. Elevated LDH levels are typically indicative of increased tumor burden and enhanced invasiveness. High LDH levels may promote tumor cell adaptation to hypoxic microenvironments, enhancing their survival and metastatic potential, thus increasing the risk of BM [23,24,25]. ALC is a direct reflection of the body’s immune function [26]. Higher ALC levels indicate stronger immune surveillance and anti-tumor immune response capabilities in patients. Lymphocytes can recognize and eliminate circulating tumor cells, preventing their colonization in distant organs [27,28,29]. Our study found that increased ALC was significantly associated with a reduced risk of BM, emphasizing the importance of strengthening immune function to inhibit BM. Maintenance therapy with immune checkpoint inhibitors has been shown to significantly extend progression-free survival (PFS) in patients with stage III NSCLC [16]. Immune maintenance therapy works by relieving immune suppression and enhancing T-cell-mediated anti-tumor immune responses, thereby inhibiting the development of minimal residual disease [30]. Our study demonstrated that patients receiving immune maintenance therapy had a lower incidence of BM, further supporting the potential role of immunotherapy in preventing BM. CEA and NSE are commonly used tumor markers, and elevated levels of these markers are associated with tumor invasiveness and metastatic potential. High CEA expression may promote tumor cell adhesion, infiltration, and angiogenesis, while elevated NSE levels suggest that the tumor may have neuroendocrine characteristics, increasing the likelihood of BBB penetration [31,32,33]. Elevations in these markers indicate a higher risk of BM. Although liver and bone metastases are not direct mechanistic contributors to brain metastasis, their inclusion in the model reflects advanced systemic disease burden. These variables help enhance risk stratification accuracy in clinical practice and may inform surveillance intensity or adjunctive therapeutic strategies.
Previous studies have primarily focused on a limited number of predictive factors. For example, Zhang et al. conducted a retrospective analysis of 637 NSCLC patients undergoing surgical treatment, identifying four independent risk factors through Cox proportional hazards regression: NSE, histological type, number of metastatic lymph nodes, and tumor grade [15]. In this study, we integrated clinical pathological features (e.g., EGFR mutation, LDH levels), immune microenvironment markers (ALC), treatment factors (immune maintenance therapy), and tumor markers (CEA, NSE) into a comprehensive predictive model. This multidimensional model more comprehensively captures the complexity of BM development, improving prediction accuracy and reliability. Existing research has paid limited attention to the role of host immune status in BM. By incorporating ALC and immune maintenance therapy into the model, our study highlights the critical role of immune factors in preventing BM. This finding provides novel insights for preventing BM through immune modulation in the future. Most existing BM prediction models are based on stage IV NSCLC patients, whose median OS is relatively short, with 5-year survival rates ranging from 2 to 13% [34]. Due to the limited survival period, the potential survival benefit window for PCI is narrow, reducing the practical value of these prediction models in clinical translation. In contrast, the PACIFIC trial demonstrated that stage III patients who received CCRT followed by immune consolidation therapy had a significant median OS of 47.5 months and a 5-year survival rate of 42.9% [35]. However, despite the significant extension in survival, BM remains one of the major factors affecting prognosis in stage III patients. In this group, due to the sufficiently long survival period, high-risk patients have enough time to benefit from PCI, thereby reducing the incidence of BM. At the same time, the prolonged survival is enough to offset the potential neurotoxic risks associated with PCI. For low-risk patients, unnecessary PCI interventions can be avoided, thus reducing treatment-related side effects and improving quality of life.
This study has several limitations that need to be considered. Firstly, this is a single-center retrospective study with a relatively small sample size, which may introduce selection bias and information bias. This study design may limit the external generalizability and applicability of the results. Future multi-center, large-sample prospective studies are needed to validate the reliability and stability of the model. Secondly, although molecular alterations such as ALK rearrangements are known to be associated with a higher propensity for BM in NSCLC, the number of ALK-positive patients in our cohort was extremely low (n = 2). As such, ALK status was not included in the final model due to insufficient statistical power. This may limit the model’s applicability in patient populations with a higher prevalence of ALK alterations. Additionally, our model primarily relies on baseline clinical pathological features and routine laboratory indicators, without incorporating molecular characteristics such as circulating tumor DNA (ctDNA) or tumor mutation burden (TMB), despite evidence that dynamic changes in ctDNA have been shown to be associated with BM risk [36]. Therefore, future studies should incorporate additional molecular and immunological markers and employ advanced modeling techniques such as machine learning to develop a more comprehensive and precise BM risk prediction model.