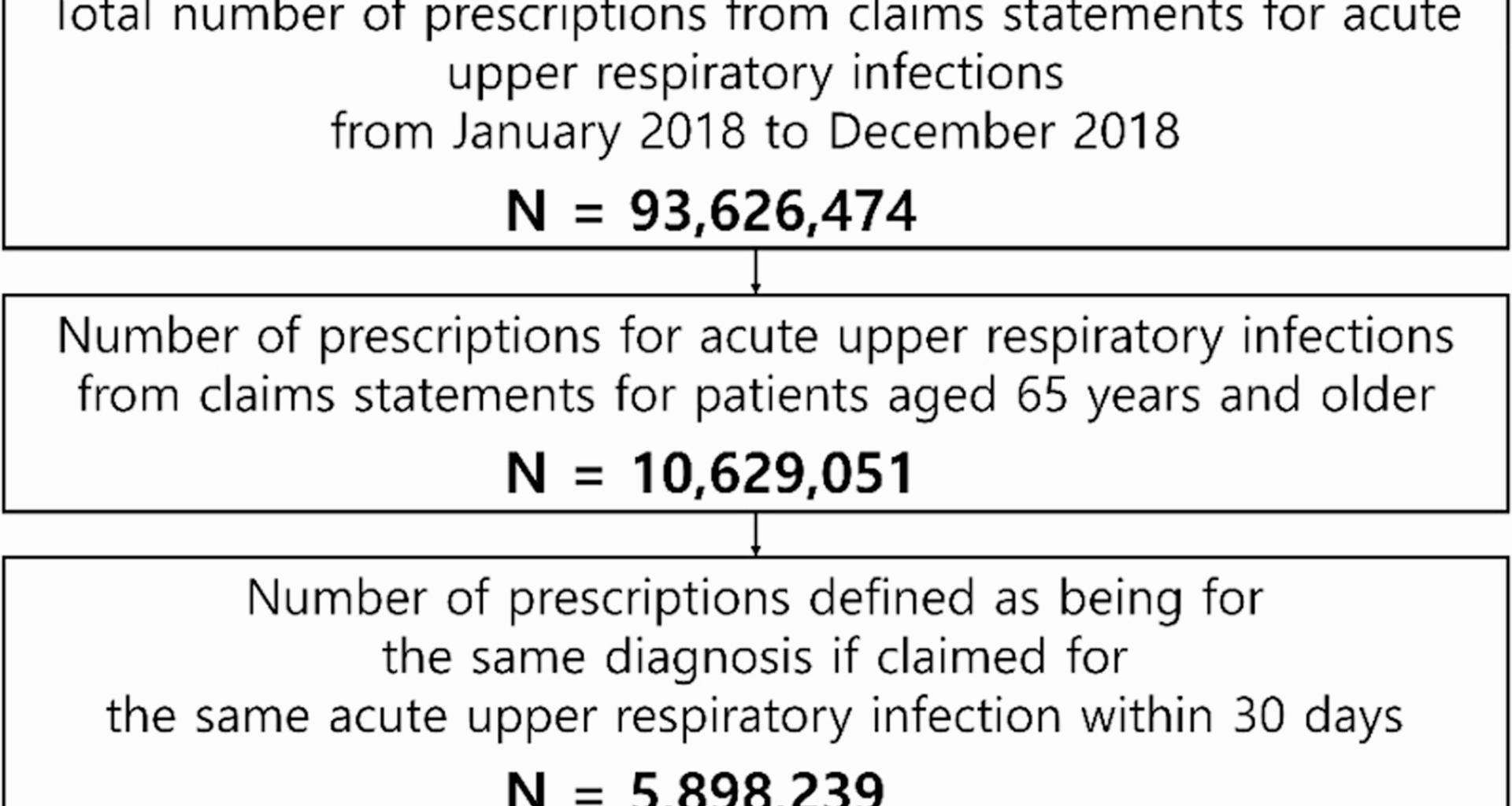
In this study 34.4% of all prescription units for patients with acute URI contained antibiotics during a 1-year period in the population aged ≥ 65 years. Additionally, 7.1% of all acute URI prescription units were associated with pneumonia. A previous cohort study of 185,014 low-risk older patients with acute URIs indicated that antibiotics were prescribed in 46.0% of those patients, categorized as common colds, acute bronchitis, acute sinusitis, or acute laryngitis [12]. Another prior claims data analysis revealed that among adults aged 20–75 years with acute URIs, the antibiotic prescription rate was 49.9% in 2008 and decreased to 39.0% in 2018 [13]. In that cohort, 35.1% of antibiotic-containing prescriptions were for acute URIs in the older demographic aged 65–75 years [13].
Previous cohort studies with the general population that documented pneumonia incidences and risk factors revealed men are 1.4 times more likely to develop pneumonia than women [14]. Several investigations noted a higher incidence of pneumonia in men compared to women, with older men having a particularly high risk [15,16,17]. This increased incidence of pneumonia in men persists even among those diagnosed with acute URIs. Our study, which focused on older patients with acute URIs, also showed that men had a higher likelihood (OR 1.192) of being diagnosed with pneumonia compared to women.
A Korean study that analyzed antibiotic prescriptions for pediatric patients with acute URIs reported the highest antibiotic prescription rate (95.8%) in primary clinics, with decreasing rates in secondary and tertiary hospitals. Pediatrics was the specialty in which the most antibiotics were prescribed, followed by internal medicine, otolaryngology, family medicine, and surgery [18]. In our study, primary hospitals accounted for 92.3% of all prescription units. Otolaryngology had the highest prescription rate among all medical specialties, followed by internal medicine, general medicine, family medicine, and surgery. These results are similar to those of a previous study with children; differences in medical specialties were possibly due to the different ages of the target population [18]. In that same study, antibiotics were prescribed in the following order among pediatric patients with acute URIs: penicillin derivatives, 2nd cephalosporins, macrolides, 3rd cephalosporins, and 1 st cephalosporins [18]. The same order was determined in our study, except that more quinolone antibiotics were prescribed than 1 st cephalosporins, which may reflect the limited use of quinolone antibiotics for children.
Considering that viral pathogens primarily cause acute URIs, antibiotics are generally ineffective, and their overuse can contribute to antimicrobial resistance [5]. Governmental efforts to manage resistance, such as Antimicrobial Stewardship campaigns and assessments of antibiotic prescribing appropriateness, have led to a decline in antibiotic prescribing rates [19]. Nonetheless, antibiotics are still prescribed for acute URIs, often to prevent potential clinical deterioration [20].
Unlike studies involving younger populations, relatively few investigations have evaluated the association between antibiotic prescriptions and specific clinical outcomes in older adults. A systematic review of patients with sore throats reported reductions in symptoms and suppurative complications among those prescribed antibiotics, although the sample size was limited [21]. Conversely, other studies have suggested that antibiotic use may be associated with an increased risk of pneumonia [22] and higher incidence rates among patients frequently prescribed antibiotics [23]. One study reported that the odds of pneumonia were 1.26 times higher in those who received antibiotics [14]. In our study of older adults with acute URIs, antibiotic prescription was independently associated with higher odds of pneumonia diagnosis (OR = 1.30).
Our results differed from a retrospective cohort study of primary care patients in the United Kingdom, which investigated the relationship of the incidence of pneumonia and serious infectious diseases with antibiotic prescription, including conditions such as otitis media, lower respiratory tract infections, and URIs [3]. Although pneumonia incidence in patients aged ≥ 65 years prescribed antibiotics for lower respiratory tract infections was relatively low 1 month later (1.5% vs. 4.0%) [3], our study observed a higher rate of pneumonia diagnosis in patients with acute URIs who received antibiotics (8.1% vs. 6.6%). This discordance may be due to differences in the inclusion criteria; that is, URIs versus lower respiratory tract infections.
COPD is a significant risk factor for pneumonia [24], with patients having a 2.18-fold increased risk compared to those without COPD [13]. Heart failure is also an independent risk factor, with a 4.11 times higher pneumonia risk [13, 25]. Cancer was associated with a 2.18 times higher risk of combined pneumonia compared to individuals without cancer [13]. Similarly, we found that comorbidities such as ischemic heart disease, heart failure, COPD, diabetes, and cancer were associated with higher odds of pneumonia diagnosis in patients with acute URIs. These findings underscore the importance of accounting for underlying chronic conditions when assessing antibiotic use in this population and support the need for more cautious prescribing practices in older adults, who often present with multiple comorbidities.
This study has several limitations. First, in some cases of acute URIs, bacterial etiologies may warrant antibiotic treatment, and both antibiotic prescribing and pneumonia diagnoses may be more frequent among patients with more severe clinical presentations. However, because our analysis relied on administrative claims data, we were unable to capture clinical details such as symptom severity or diagnostic confirmation to directly assess these factors. Second, pneumonia diagnoses were identified based on ICD-10 codes recorded at any point during the one-year study period, without confirmation that pneumonia events occurred after the URI episode. As such, the temporal sequence between URI diagnosis, antibiotic prescription, and pneumonia onset could not be definitively determined. This limitation restricts our ability to draw causal inferences from the observed associations. Third, although we assessed prescription records, we could not verify whether the prescribed antibiotics were actually dispensed or taken by the patients. Finally, important contextual factors such as smoking status, immunocompromised conditions, socioeconomic status, and characteristics related to healthcare providers were not available in the claims data. The absence of these variables may lead to residual confounding and limits the generalizability and interpretability of the findings.