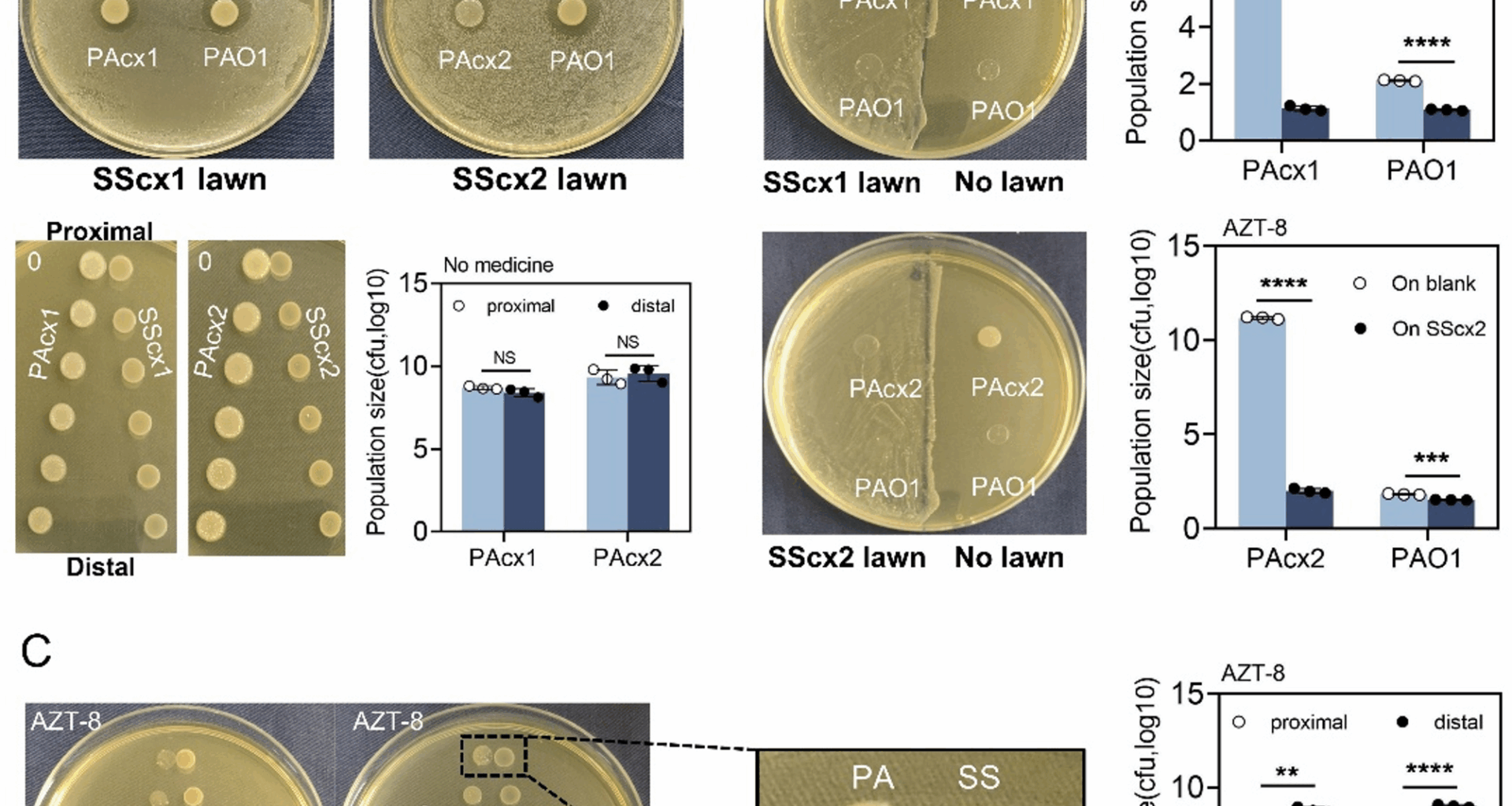
Treatment with aztreonam affects the interspecific interaction of bacteria
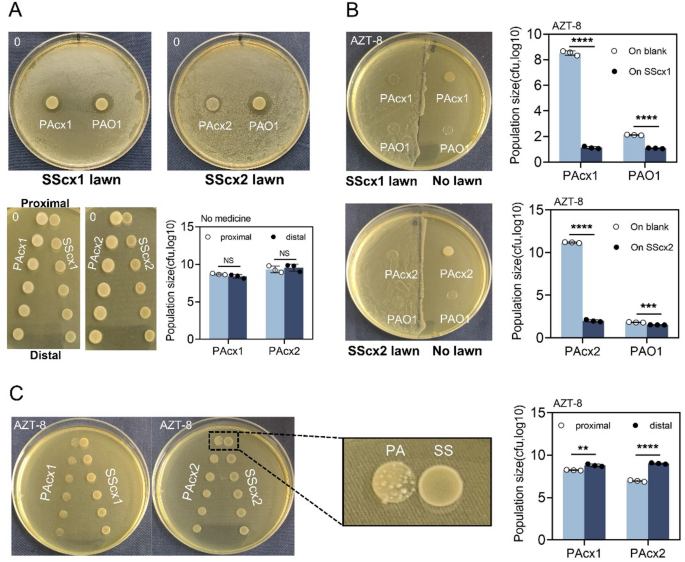
The interaction between P. aeruginosa and S. salivarius was first revealed on BHI agar plates. Regardless of whether examining PAcx1, PAcx2, or the model strain PAO1, all strains grew normally on S. salivarius lawns and produced varying degrees of inhibition zones (Fig. 1A, Fig. S1A). In contrast, S. salivarius was unable to grow on P. aeruginosa (Fig. S2). In previous experiments, we conducted MIC testing using commonly used antibiotics on two pairs of strains (Table. S2). To simulate the differential effects of antibiotics on the complex pulmonary microbiota, we selected aztreonam, a β-lactam antibiotic with significant resistance differences between P. aeruginosa and S. salivarius, as the background antibiotic for further research. At 8 µg/mL aztreonam (sub-MIC for P. aeruginosa), the growth of S. salivarius was unaffected, whereas the initial growth of P. aeruginosa was delayed. However, within 24 h, P. aeruginosa reached a bacterial density nearly equivalent to that observed in the blank control (Fig. S3). Next, we tested the effect of different gradient concentrations of aztreonam on the growth of P. aeruginosa, with and without S. salivarius lawns, near the MIC of P. aeruginosa (16 µg/mL) (Fig. S4). Interestingly, by contrast, P. aeruginosa was almost unable to grow on S. salivarius with high antibiotic resistance in the presence of sub-inhibitory concentrations of aztreonam (8 µg/mL). Compared to its growth on no lawn, P. aeruginosa grew poorly on the S. salivarius lawn and failed to produce an inhibition zone (Fig. 1B). Under the same drug-treated conditions, the growth of clinical P. aeruginosa strains on S. salivarius ATCC 27,945 remained suppressed (Fig. S5). However, this inhibitory effect was significantly weaker than that observed with clinically isolated S. salivarius strains (Fig. S4). The stronger inhibition by clinical S. salivarius strains likely reflects their evolutionary adaptation in COPD airways.
Interaction between P. aeruginosa and S. salivarius under the intervention of aztreonam. A PAcx1 and PAcx2 inhibit the growth of SScx1 and SScx2 on blank BHI agar plates, as indicated by the formation of inhibition zones on the corresponding S. salivarius lawn. Notably, S. salivarius showed negligible influence on P. aeruginosa colony development in both adjacent and distant regions. B Comparison of the growth of clinical P. aeruginosa strains and PAO1 on clinical S. salivarius lawns and non-lawn areas on aztreonam plates (8 µg/mL). Compared to growth in non-lawn areas, the clinical P. aeruginosa strains exhibit poorer growth on S. salivarius lawns. P. aeruginosa colonies are extracted, suspended in 1.0 mL of sterile saline, serially diluted, and plated for CFU enumeration. C Proximal and distal colony analysis of PAcx1, PAcx2, SScx1 and SScx2 grown on plates containing aztreonam (8 µg/mL). P. aeruginosa colonies are extracted from proximal and distal sites, suspended in 1.0 mL of sterile saline, serially diluted, and plated for CFU enumeration. AZT: aztreonam. The presented data represent the mean ± standard deviation (SD) of three independent experiments, with statistical comparisons performed using a two-tailed paired t-test. **, p p p
To further investigate, a pairwise proximity assay was designed to test the mutual effects of the extracellular products of the two species. The two species were grown on BHI plates at increasing distances from proximal to distal regions, and CFU quantification was performed on the proximal and distal colonies of P. aeruginosa and S. salivarius. Under antibiotic-free conditions on BHI agar plates, S. salivarius exhibited no significant inhibitory or stimulatory effects on either proximal or distal P. aeruginosa colony formation (Fig. 1A), but with the addition of 8 µg/mL aztreonam, S. salivarius inhibited the growth of the proximal P. aeruginosa colonies (Fig. 1C). Furthermore, we further confirmed that aztreonam significantly influences the interspecies interaction by growing the two species on each other’s extracellular products. P. aeruginosa, which typically produces varying levels of anti-Streptococcus molecules on blank BHI plates, showed almost no effect on S. salivarius when cultured on aztreonam-containing plates. In contrast, S. salivarius, which initially had no effect on P. aeruginosa, exhibited antimicrobial inhibition of proximal P. aeruginosa in the presence of aztreonam (Fig. S6). It was suggested by these findings that the growth of both species could be inhibited by each other, but the competitive advantage was dependent on the presence of aztreonam.
Aztreonam reverses the dynamic competition between P. aeruginosa and S. salivarius
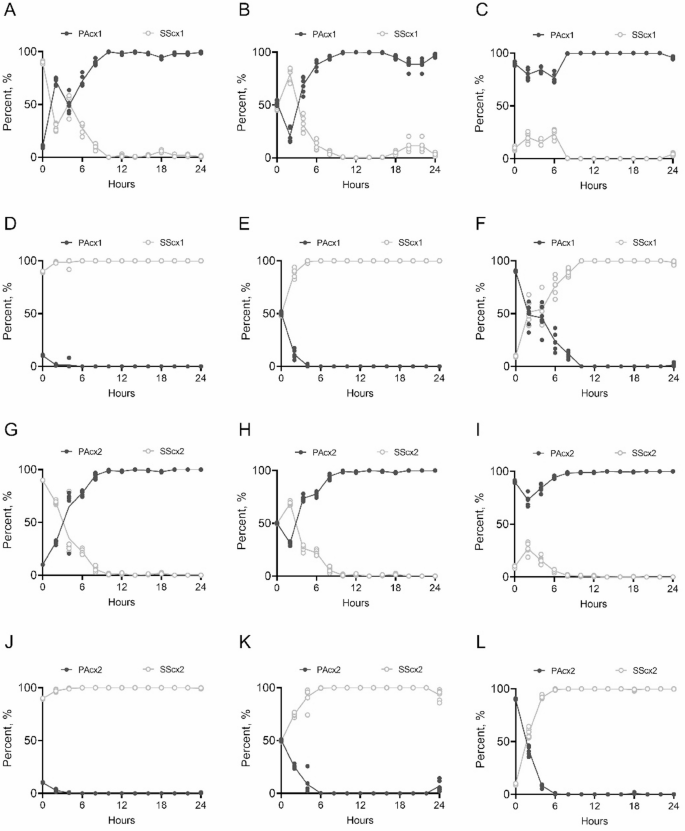
After exploring the static interaction between P. aeruginosa and S. salivarius on plates, we began monitoring their dynamic interaction. Using mixed bacterial cultures with different initial ratios under various growth conditions, we monitored the final P. aeruginosa to S. salivarius ratio at different time points to assess changes in the competitive dynamics under varying conditions. The laboratory has developed a relatively mature method for dynamic bacterial game analysis [10]. In brief, the ratio of the two bacterial species in sterile plastic tubes is measured every 2 h during the 24-hour antibiotic efficacy period.
On blank plates, regardless of the initial ratio of the mixed bacterial cultures, both PAcx1 and PAcx2 invaded SScx1 and SScx2 within a short period (8 h) with overwhelming competitive advantage (Fig. 2A-C, G-I), consistent with the activation time of P. aeruginosa’s QS system [24]. Interestingly, at sub-inhibitory concentrations of aztreonam (which had minimal effect on P. aeruginosa’s growth density) (Fig. S3), regardless of the initial ratio, SScx1 and SScx2 gained the upper hand and successfully invaded PAcx1 and PAcx2 (Fig. 2D-F, J-L).
The interaction dynamics of clinical P. aeruginosa and S. salivarius at varying initial mixing ratios over 24 h. The final proportions at different time points are assessed during co-culture on blank BHI plates and BHI plates supplemented with 8 µg/mL aztreonam. The co-culture dynamics of PAcx1 and SScx1 at mixing ratios of 1:9 (A, D), 1:1 (B, E), and 9:1 (C, F) on blank BHI plates (A, B, C) and aztreonam-containing plates (D, E, F). The co-culture dynamics of PAcx2 and SScx2 at mixing ratios of 1:9 (G, J), 1:1 (H, K), and 9:1 (I, L) on blank BHI plates (G, H, I) and aztreonam-containing plates (J, K, L). Samples are collected at 2-hour intervals over a 24-hour period. The data presented represent the mean (lines) of six independent replicates (circles)
Extracellular products of S. salivarius inhibit QS virulence gene expression in P. aeruginosa
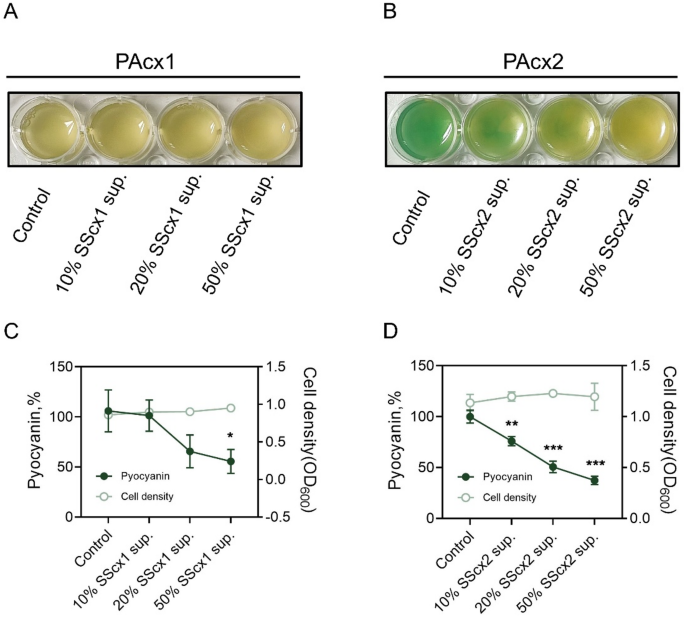
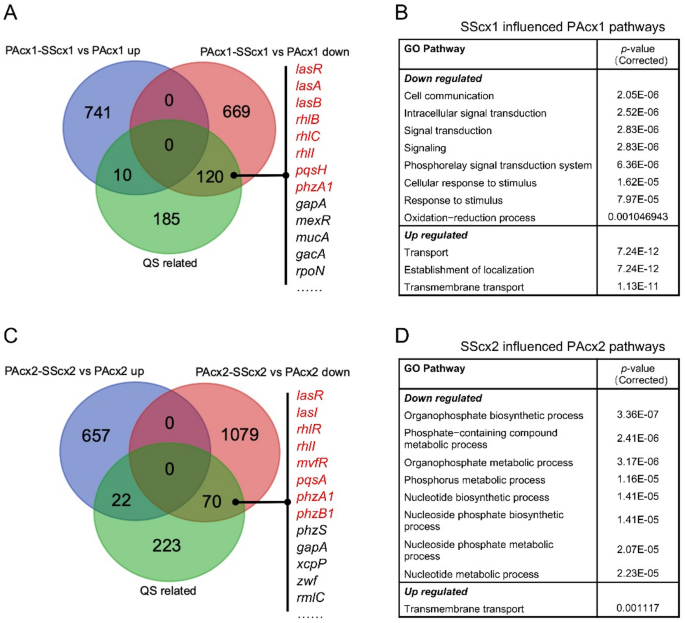
P. aeruginosa secretes various virulence factors (such as elastase, exotoxins, rhamnolipids, pyocyanin, and lectin A) to adapt to the host’s adverse environment [25, 26]. All of these virulence factors are regulated by cell density and controlled by three key QS systems (las, rhl, and pqs) [27]. Among these virulence factors, pyocyanin is one of the key contributors to disease severity, tissue damage, and organ dysfunction [28]. Notably, the production of pyocyanin is regulated by multiple QS systems and is directly controlled by mvfR (pqsR) [29, 30]. To evaluate the impact of S. salivarius extracellular products on P. aeruginosa virulence, different gradients of cell-free S. salivarius culture supernatant were added to P. aeruginosa cultures. The results showed that while increasing the S. salivarius supernatant concentration negligible impact on P. aeruginosa growth density, it significantly inhibited pyocyanin production by P. aeruginosa (Fig. 3), particularly in the lasR-mutant strain PAcx2 (Fig. 3B and D). RNA sequencing (RNA-seq) results identified significantly differentially expressed genes from P. aeruginosa treated with S. salivarius supernatant and mapped them to the list of QS activation genes reported for P. aeruginosa by Schuster et al. [21]. The results showed that after treatment with 50% S. salivarius supernatant, PAcx1 had a reduction of 120 QS-related genes, while PAcx2 showed a decrease of 70 QS-related genes compared to the untreated group (Fig. 4A and C). Both P. aeruginosa strains showed a reduction in central regulatory genes (las, rhl) and key genes involved in pyocyanin biosynthesis (phzA1). Additionally, PAcx2 exhibited inhibition of mvfR, phzS, and other key genes controlling pyocyanin synthesis. Furthermore, downregulated genes were enriched in GO pathways, with PAcx1 primarily downregulated in pathways related to cell communication, intracellular signaling, propionate metabolism, and amino acid degradation (Fig. 4B). Due to the poor oxidative stress tolerance of lasR-mutant strain PAcx2, downregulated genes were primarily enriched in oxidative phosphorylation, cell motility (cilia and flagella), and nucleotide metabolism and synthesis (Fig. 4D). Therefore, the transcriptomic data suggest that S. salivarius extracellular products inhibit the virulence regulation of P. aeruginosa and reduce its metabolic capacity.
S. salivarius cell-free supernatant inhibits pyocyanin production in P. aeruginosa. A. Pyocyanin production in PAcx1 cultures supplemented with varying concentrations of SScx1 supernatant. B. Pyocyanin production in PAcx2 cultures supplemented with varying concentrations of SScx2 supernatant. C. Effects of SScx1 supernatant at different concentrations on pyocyanin production and cell density in PAcx1. High concentrations of SScx1 supernatant significantly reduce pyocyanin production in PAcx1 without affecting cell density. D. Effects of SScx2 supernatant at different concentrations on pyocyanin production and cell density in PAcx2. Pyocyanin production in PAcx2 is inhibited by SScx2 supernatant in a dose-dependent manner, while cell density remains unaffected. Pyocyanin absorbance at 520 nm is normalized to the corresponding cell density and further standardized against the control. Data are presented as mean ± SD from three independent experiments and compared using two-tailed unpaired t-tests. *, p p p
Under drug-free conditions, 50% of S. salivarius cell-free supernatant induced transcriptomic differences in the gene expression of P. aeruginosa. A Differential effects of SScx1 supernatant on QS-related genes in PAcx1. B RNA-seq analysis identifies significant impacts of SScx1 supernatant on GO metabolic pathways in PAcx2. C Differential effects of SScx2 supernatant on QS-related genes in PAcx2. D RNA-seq analysis identifies significant impacts of SScx2 supernatant on GO metabolic pathways in PAcx2
Aztreonam activated S. salivarius and inhibited P. aeruginosa during cross-feeding
To investigate the transcriptomic and metabolic differences between P. aeruginosa and S. salivarius under aztreonam intervention, we conducted a cross-feeding experiment [31]. The cells were cultured in BHI medium and physically separated using a 0.22 μm filter that did not allow cellular penetration. After normalizing the cell densities, both bacterial species were inoculated on the inner and outer sides of the filter membrane and cultured in either untreated or aztreonam environments. RNA sequencing results showed that after antibiotic addition, the transcriptomes of both species in the cross-feeding setup were differentially affected compared to when cultured individually.
Two sets of RNA sequencing comparisons were performed for SScx1 and SScx2: (1) with and without aztreonam intervention in individual S. salivarius cultures; (2) with and without aztreonam intervention in co-cultures of S. salivarius and P. aeruginosa. In the individual S. salivarius culture, the addition of aztreonam had minimal effect on its genome (Fig. S7A and S7B). Consistent with the previous phenotypic observations, the co-culture of P. aeruginosa under blank conditions resulted in the differential downregulation of hundreds of S. salivarius genes (Fig. S7C and S7D). Interestingly, under cross-feeding co-culture conditions, aztreonam intervention, in contrast to the drug-free condition, activated the expression of S. salivarius genes. SScx1 exhibited an upregulation of 676 genes, while SScx2 demonstrated an upregulation of 711 genes, with the degree of differential expression being significantly higher than that of the downregulated genes (Fig. S7E and S7F). Additionally, both SScx1 and SScx2 exhibited differential upregulation of growth and biosynthetic metabolic pathways, including those related to translation, transcription, protein synthesis, ribosome activity, and macromolecule production (Fig. S8A and S8B).
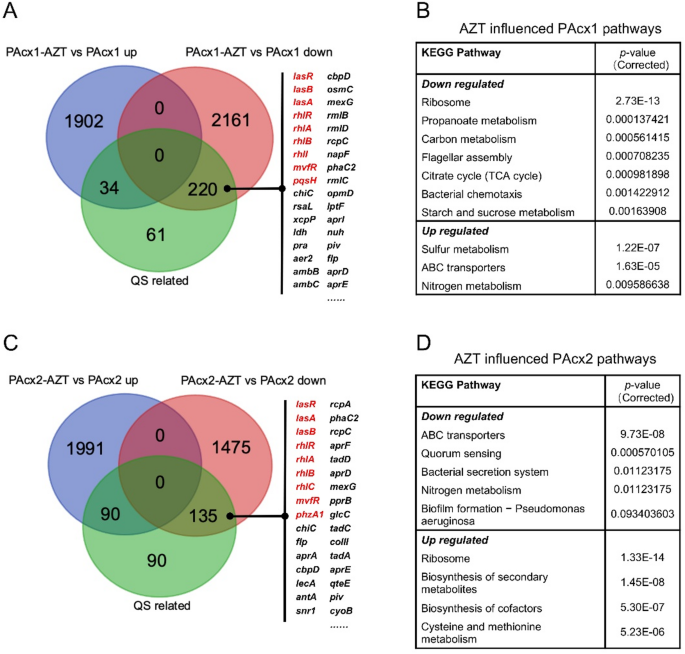
Two sets of RNA sequencing comparisons were performed for PAcx1 and PAcx2: (1) with and without aztreonam intervention in individual P. aeruginosa cultures; (2) with and without S. salivarius intervention in P. aeruginosa cultures under aztreonam treatment. In individual P. aeruginosa cultures, aztreonam treatment led to differential downregulation of 220 QS-related genes in PAcx1 and 135 in PAcx2, including the central regulatory genes lasR, rhlR, mvfR, and other associated genes (Fig. 5A and C). Additionally, it is worth noting that under antibiotic-treated conditions, the introduction of S. salivarius had a differential impact on P. aeruginosa compared to its monoculture. In PAcx1, the addition of SScx1 resulted in downregulation of 32 QS-related genes, including pqsH, which promotes the functioning of the PQS system, and phzA1, which is associated with pyocyanin production (Fig. S9A). For the lasR-mutant strain PAcx2, the lasI gene, which synthesizes the autoinducer C12 molecule (3-oxo-C12-HSL), and key genes upstream of the PQS system (pqsABCDE) were suppressed by the intervention of SScx2 (Fig. S9C). The downregulated pathways were enriched in KEGG pathways, with PAcx1 primarily showing downregulation in glyoxylate metabolism, carbon metabolism, and QS (Fig. S9B). In contrast, the lasR-mutant strain PAcx2 exhibited downregulation not only in carbon metabolism but also in several other metabolic pathways, including fatty acid biosynthesis, amino acid metabolism, and oxidative phosphorylation (Fig. S9D).
RNA-seq analysis reveals that aztreonam inhibit QS gene expression in P. aeruginosa. A Sub-inhibitory concentrations of aztreonam significantly downregulate key QS genes in PAcx1. B Sub-inhibitory concentrations of aztreonam significantly affect the metabolic pathways of PAcx1. C Sub-inhibitory concentrations of aztreonam significantly downregulate key QS genes in PAcx2. D Sub-inhibitory concentrations of aztreonam significantly affect the metabolic pathways of PAcx2. AZT: aztreonam
In summary, transcriptomic data indicate that S. salivarius can be activated by aztreonam during co-culture. For P. aeruginosa, however, regardless of whether it is cultured alone or in co-culture, and irrespective of aztreonam addition, its QS system and metabolic activity are inhibited to a certain extent.
Extracellular products of S. salivarius Reduce virulence and mortality of P. aeruginosa under aztreonam treatment
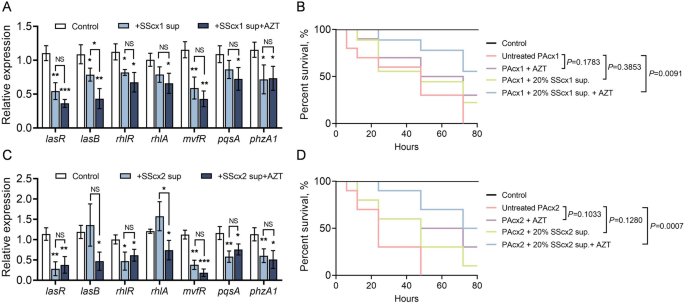
To validate the downregulation of P. aeruginosa QS genes by extracellular products of S. salivarius and under aztreonam treatment, qPCR was conducted with three control groups targeting P. aeruginosa core QS genes. Stimulation by S. salivarius supernatant almost completely inhibited the expression of key QS system-regulated genes in P. aeruginosa (Fig. 6A and C). Furthermore, in the presence of aztreonam, the S. salivarius supernatant exhibited even greater toxicity toward P. aeruginosa, significantly further reducing QS gene expression. Notably, in the qPCR results of PAcx2, while the SScx2 supernatant alone showed no significant effect on certain QS genes, aztreonam intervention led to a moderate suppression of genes such as lasB and rhlA (Fig. 6C). This observation aligns with the previous transcriptomic data. Overall, the qPCR results further confirmed that the downregulation of P. aeruginosa QS-related genes is associated with S. salivarius involvement and aztreonam treatment.
Reduction in the virulence and killing efficiency of P. aeruginosa by extracellular products of S. salivarius under aztreonam treatment. A qPCR analysis of changes in key QS gene expression levels in PAcx1 upon the addition of 20% SScx1 supernatant and 8 µg/mL aztreonam. B SScx1 supernatant significantly reduces PAcx1 lethality under aztreonam treatment. Survival rates of nematodes exposed to PAcx1 with or without supplementation of 20% SScx1 supernatant and/or 8 µg/mL aztreonam. C qPCR analysis of changes in key QS gene expression levels in PAcx2 upon the addition of 20% SScx2 supernatant and 8 µg/mL aztreonam. D SScx2 supernatant significantly reduces PAcx2 lethality under aztreonam treatment. Survival rates of nematodes exposed to PAcx2 with or without supplementation of 20% SScx2 supernatant and/or 8 µg/mL aztreonam. Panels A and C present mean values ± SD from three independent experiments. Statistical significance was determined using a two-tailed unpaired t-test: *, p p p B and D use the log-rank (Mantel-Cox) test to compare survival curves. Control groups consisted of nematodes fed E coli OP50 under identical plate conditions
Finally, we utilized a C. elegans model to test the impact of S. salivarius extracellular products in the presence of aztreonam on the lethality of P. aeruginosa. Untreated PAcx1 and PAcx2 exhibited markedly higher toxicity toward C. elegans, with nearly complete mortality recorded within 72 h (Fig. 6B and D). In contrast, treatment groups exposed to either aztreonam or S. salivarius supernatant showed no significant differences but exhibited consistently lower mortality rates compared to the untreated group, with survival rates exceeding 0% at 72 h. Interestingly, in the presence of aztreonam, the addition of S. salivarius supernatant markedly improved nematode survival, substantially reducing the lethality of P. aeruginosa. These findings indicate that both S. salivarius and aztreonam can suppress the virulence of P. aeruginosa. Notably, in the presence of aztreonam, S. salivarius exhibits enhanced inhibitory effects on P. aeruginosa, leading to a significant reduction in its lethality toward nematodes.