Prostate cancer (PC) is the second most common cancer among men worldwide, and it is also the fifth most fatal malignancy among men globally [1]. Monitoring prostate-specific antigen (PSA) levels during follow-up is a widely accepted and cost-effective method for detecting prostate cancer relapse [8].
Ga68-PSMA PET/CT was recently recommended for use in patients with PC. Multiple studies have found it to have superior diagnostic accuracy, specificity, and sensitivity compared to other traditional imaging methods for confirming biochemical recurrence in large patient populations with recurrent PC [4, 5].
This research aimed to evaluate the role of Ga68-PSMA in the biochemical recurrence of prostate cancer.
In the current study, the recurrence was higher among the high socioeconomic class.
This result is in concordance with that of Coughlin [9], who stated that socioeconomic factors play a role in influencing the risk of prostate cancer. Incidence rates of prostate cancer tend to be positively associated with higher socioeconomic status. In comparison, lower socioeconomic status is often linked to an increased risk of lower survival rates among individuals diagnosed with prostate cancer [10,11,12].
The residence of the patient is another point here in the current study, where urban residency was found to be associated with a higher recurrence of prostate cancer than rural residency. This finding is in disagreement with that of Stolzenbach et al. [11], who found that individuals living in rural areas have a higher incidence of prostate cancer and its complications. Our result represents a small sample size and could be related to the cultural aspects of urban areas that may differ from that of Stolzenbach et al. [11] study.
In the present study, radical prostatectomy was the surgical option for 73.3% of cases. Twenty percent of our patients received postoperative radiotherapy with a mean PORT dose (Gy) of 65.33 ± 3.07. The objective of post-prostatectomy radiation therapy is to minimize the risk or prevent the recurrence of cancer in the prostate bed [13].
ADT was used in 56.7% of our patients, with a mean duration of therapy of 34.54 ± 24.4 months. In a study done by Dorff et al. [14], they found that two years of ADT was likely a significant contributor to improved outcomes after radical prostatectomy. The utilization of PSA for identifying recurrent disease has been in practice for over 35 years [15].
In the current study, the mean of the biochemical recurrence-free interval was 56.1 ± 34.03 months, with the mean PSA value in the first measurement and the confirmatory level being 34.62 ± 120.08 and 35.81 ± 123.8 (ng/ml), respectively. These results matched those reported by Jemal et al. [16]. The rising levels in the current study are in agreement with those of Fossati et al. [17], who mentioned that an increasing PSA level was the initial indicator of disease progression following radical prostatectomy (RP).
Regardless of the BCR definition, it should not be used as a landmark to start treatments [18], so in the current study, we used Ga68-PSMA PET/CT to further evaluate BCR.
In the current study, surgical bed recurrence occurred in 30% of patients. In addition, local LN deposits were found in 33.3% of patients, while distant LN deposits were found in 23.3%. Furthermore, bone metastasis was the most common distant metastasis found in 30% of our patients.
In this domain, the results of the current study were plotted against the results from other centers where Nasser et al. [18] found that prostate bed recurrence was observed in 15% of patients, 29% of patients had local lymph node recurrence, and 13% of patients had distant lymph node metastasis. On the other hand, Ilhan et al. [19] reported that local recurrence was observed in 80% (78 out of 97 cases), while pelvic lymph node metastases were identified in 38% (37 out of 97 cases).
Prostatectomy bed recurrence (local relapse) occurred in 30%, regional relapse occurred in 33.3%, and distant relapse was recorded in 30% of our studied patients. These results were greater than those previously reported by Calais et al. [20] and Emmett et al. [21], who found that the frequency of prostatectomy bed recurrence was 17.5% and 23%, respectively. On the other hand, our results were lower than those reported by Moore et al. [22], who found that the recurrence pattern after RP was predominantly local (≈60%).
The differences between these and our study results could be attributed to the time our patients sought medical follow-up and the difference in several studied patients.
Regarding the type of relapse, this study revealed that bone relapse only was the most common distant relapse and was detected in (16.6%) of patients, followed by bone and visceral relapse reported in (13.3%) of the studied patients.
These findings are nearly similar to those reported by Szigeti et al. [23], who found bone metastases (BM) in 14.8% (12/81) of the patients on Ga68-PSMA—PET CT; 6 patients had BM in the pelvic region.
In the present study, regional relapse was the most common relapse (representing 23.3% of patients); it is worth mentioning that a group of patients in our study developed both local relapse and distant relapse (representing 13.3% of patients) while a group of patients developed local relapse only (representing 10% of patients). Our findings were in concordance with those reported by Von Eyben et al. [24], who found that 30% of patients had a regional relapse, followed by distant relapse in 20% and local relapse in 13% of patients.
PET metrics include qualitative assessment indices, such as the maximum standardized uptake value (SUVmax). In the current study, we studied this point about SUVmax, and the highest SUVmax mean was 45 in a patient with rectal invasion relapse, and the lowest SUVmax mean was 5.12 ± 0.81 in patients with lung deposits. This came in agreement with Eder et al. [25], who reported that the lowest SUVmax mean was 2.49 ± 0.27 in patients with lung deposits, while they found that the highest SUVmax mean was 139.4 ± 21.4 in patients who had kidney relapse.
Pepe et al. [26] stated that Ga68-PSMA PET/CT, utilizing an SUVmax cutoff of 8, exhibited high accuracy in diagnosing prostate cancer (PC), achieving 100% accuracy in the presence of Gleason Grade (GG) ≥ 3. This suggests a favorable cost–benefit ratio, positioning it as a comprehensive and efficient procedure for both the diagnosis and staging of high-risk prostate cancer.
In our study, the PSA level was significantly higher in patients who had relapses than those without (55.46 ± 150.9 vs 0.24 ± 0.04). This finding is in concordance with that of David and Leslie [27] who reported that PSA levels were higher in recurrence cases than those without recurrence, and the elevation in PSA levels typically serves as the initial indicator of recurrent prostate cancer.
Szymaniak et al. [28] reported that a rising PSA level after prostatectomy indicates PC recurrence, a finding that supports the current study’s results. In addition, Mapelli et al. [29] consider rising PSA a predictor of prostate cancer recurrence. These findings agree with ours.
In the current study, prostate cancer patients with regional relapse had the highest first and confirmatory PSA levels (85.14 ± 4.95 and 88.1 ± 225.3 ng/ml, respectively), while patients with local relapse had the lowest first and confirmatory PSA levels (3.68 ± 4.95 and 3.93 ± 5.09 ng/ml, respectively).
This aligns with the findings of Oyama et al. [30], who stated that higher PSA level in PC patients was associated with a high likelihood of regional relapse.
Our results showed that Ga68-PSMA PET/CT at a cutoff level ≥ 0.3 ng/ml could detect early prostate cancer relapses with 94.7% sensitivity, 81.8% specificity, 90% PPV, and 90% NPV. The accuracy of early diagnosis of prostate cancer relapse was 90%.
Our results in this domain agreed with those of Fendler et al. [4], who reported that even when serum levels of PSA are low, Ga68-PSMA PET/CT can accurately detect prostate cancer relapse. Furthermore, our findings are consistent with those of Haidar et al. [31], who reported that Ga68-PSMA PET/CT successfully detected prostate cancer recurrence in patients undergoing biochemical recurrence (BCR) and that there was a strong correlation between the PET scan’s positivity and the patient’s PSA level on the day of the scan.
Consistent with our findings, a surgical series in a meta-analysis on diagnosing lymph node metastases using Ga68-PSMA PET/CT found a significant specificity rate of 97% and a moderate sensitivity rate of 60% [24].
Other researchers have investigated the sensitivity of Ga68-PSMA PET/CT, revealing high sensitivity and excellent specificity for detecting prostate cancer, particularly in cases of recurrent disease, even at very low levels of PSA ranging from 0.2 to 0.5 ng/ml [32, 33]. These studies supported our findings.
To compare our findings in sensitivity and specificity of Ga68-PSMA PET/CT, we found that another study by Kwan et al. [34] found a high sensitivity of Ga68-PSMA PET/CT for the detection of post-radical prostatectomy relapse: 81.2%.
In addition, Maurer et al. [35] mentioned that the applications of Ga68-PSMA PET/CT are varied, encompassing improved detection of new metastases and the identification of cancer in cases of biochemical recurrence.
In another study done by Yan et al. [36], they demonstrated that Ga68-PSMA PET/CT had a sensitivity of 60%, a specificity of 91%, and an accuracy of 83% for detecting pelvic lymph node metastasis. In a more extensive study involving 319 patients and analyzing a mixed patient population retrospectively, Ga68-PSMA PET/CT demonstrated a sensitivity of 76.6% and a specificity of 100% [37].
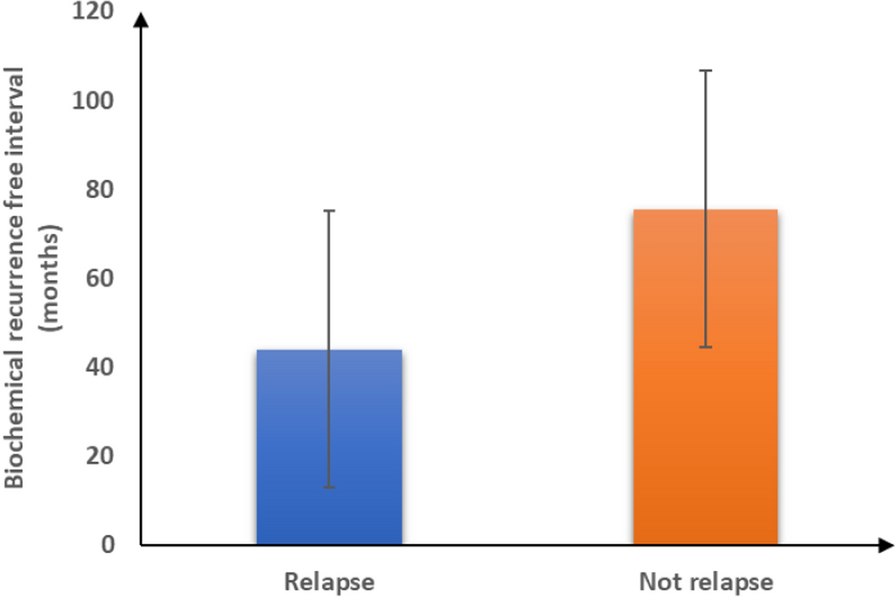
In the current study, the biochemical recurrence-free interval among patients with no relapse was significantly longer when compared with that of patients who had relapse (75.54 ± 31.06 vs 44.84 ± 31.07 months, p = 0.01). In addition, we found that the mean biochemical recurrence-free interval for patients who had PSA 0.3 ng/ml (119 vs 50 months, p = 0.001). In this regard, our findings matched those of Freedland et al. [38], who reported that early biochemical recurrence (BCR) has been linked to a heightened risk of recurrence and death from prostate cancer.
In another study, a reduced period from surgery to biochemical recurrence (BCR) has been reported as an independent predictor of metastatic progression, relapse, cancer-specific mortality, and overall mortality [39].
Our retrospective study has several limitations: our relatively small (30 patients). This could limit the generalization of the current results. Patients whose PSMA PET/CT results were positive or negative should have their long-term outcomes evaluated later; our group is still being followed up. There should be a plethora of prospective randomized trials because the data that are now available are insufficient.