Traditional fermented foods vary by raw ingredients and fermentation methods
We collected 90 samples of 24 different types of fermented foods from Nepal (n = 64), South Korea (n = 20), Ethiopia (n = 5), and Kazakhstan (n = 1) that were traditionally prepared for household consumption using diverse fermentation techniques (Fig. 1a, Supplementary Table 1). These ferments encompass 53 vegetables, 13 legumes, 5 cereals, 14 dairy and 5 animal products (meat or seafood). The vegetable-based ferments include achars (n = 24), gundruk (n = 12), sinki (n = 2), and taama (n = 4) from Nepal, kimchi (n = 5), oiji (n = 1), chilli paste (n = 1), pickled plum (n = 1), and plum juice (n = 1) from South Korea, as well as awaze (n = 1) and datta (n = 1) from Ethiopia. The cereal-based ferments included Ethiopian injera (n = 1) and difo dabo (n = 1) as well as the alcoholic beverage chhyang (n = 1) and its amylolytic starter marcha (n = 2) from Nepal. Fermented legumes consisted of Nepali masyaura (n = 4), as well as fermented soybean pastes (n = 5), and soy sauces (n = 4) from South Korea. The dairy based ferments included 1 cheese sample from Kazakhstan, Ethiopian ayib (n = 1), and Nepali dahi (n = 4), chhurpi (n = 3), and soft cheese (n = 5) samples. We also included fermented meat products from Nepal called sukuti (n = 3) and fermented seafood (n = 2) from South Korea. The italicized words throughout the manuscript represent the names of fermented foods in their respective languages.
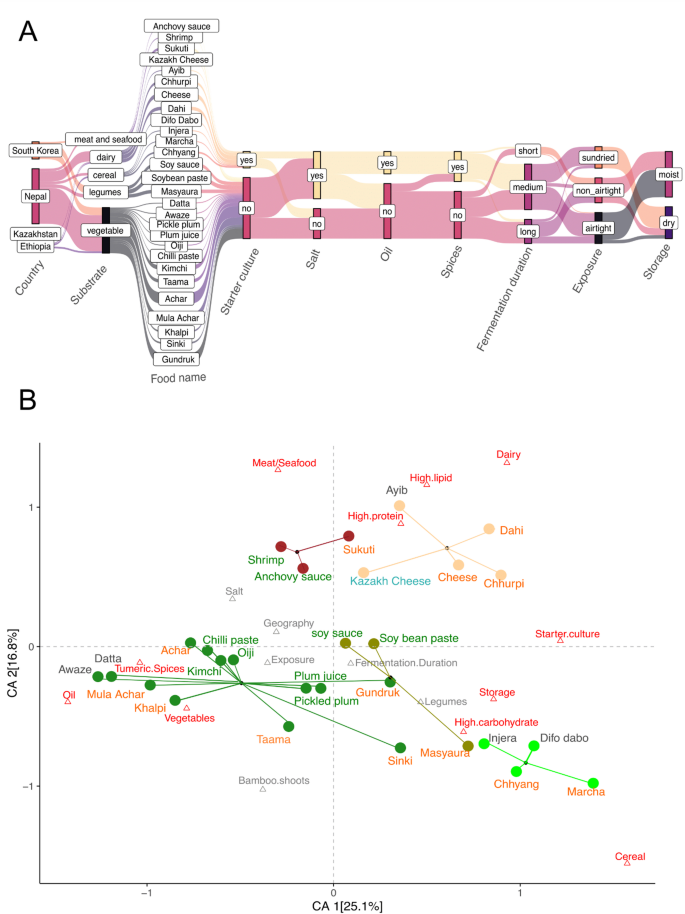
Diversity of raw ingredients and preparation methods used in traditional fermentation. We sampled 90 samples of 24 unique fermented foods from Nepal, South Korea, Ethiopia, and Kazakhstan. A Key parameters that vary during preparations of traditional fermented foods. Generally, Nepali achars, khalpi, mula achar and South Korean kimchi, oiji, and fermented chilli paste are prepared from garden vegetables marinated in turmeric, spices, oil, and salt that are spontaneously fermented by tightly packing and sealing in airtight containers. Nepali gundruk (mustard greens), sinki (radish), and taama (bamboo shoots) along with pickled plum and plum juice from South Korea, and datta and awaze from Ethiopia also rely on spontaneous fermentation. Legume based Nepali fermented foods such as masyaura, which are nuggets of ground black lentil paste mixed with taro, yam, or colocasia leaf and South Korean soy sauce and soybean paste are prepared without use of spices or oil. Gundruk, sinki, and masyaura are sun dried before storing at room temperature for up to one year. Fermented dairy products from Nepal, including dahi (yogurt), soft cheese, and chhurpi (dried and hardened cheese), Ethiopian ayib, and Khazakhi cheese are fermented using starter cultures. The cereal based fermented foods of Ethiopia included injera and difo dabo as well as the Nepali alcoholic beverage chhyang, which uses the amylolytic starter marcha, also rely on starter cultures. Fermented meat such as sukuti is marinated in spices and spontaneously fermented before sun drying. Similarly, South Korean fermented seafoods such as anchovy sauce and shrimp are fermented in salty brine solution. Methods of preparation are consistent for food samples from the same location and type, although minor variations in specific aspects of the preparation process, such as the amount of salt and spices used, fermentation duration, and duration of exposure to the environment may be anticipated due to differences in personal preferences. B Correspondence analysis of 17 abiotic variables associated with the different traditional fermented foods included in this study. Each food is represented by a circle colored by substrate: vegetables (dark green), dairy (orange), meat/seafood (maroon), cereal (lightgreen), and legumes (olive). The corresponding text labels are colored by the country of origin: Nepal (orange), South Korea (green), Ethiopia (grey) and Kazakhstan (blue). Each triangle represents an abiotic factor associated with food preparation and macronutrient profile. Factors in red contribute most to the top two dimensions in the CA. The first dimension of the correspondence analysis (CA1) primarily differentiates the spontaneously fermented vegetables and animal products mixed with oil, salt, and spices from foods fermented using starter cultures such as protein-rich dairy products (dahi, soft cheeses, and chhurpi) and cereal-based alcoholic beverage chhyang along with its starter marcha. Fermented plants (vegetables and cereals) differ from fermented animal-based products (meat, seafood, and dairy) along CA2
Using current literature, we identified 17 variables describing macronutrient profiles and fermentation processes that distinguish the traditional fermented foods included in this study [44]. Multivariable analysis of these descriptors revealed that fermented foods from different regions of the world cluster together based on substrate type and preparation methods (Fig. 1b). Notably, plant-based foods such as fermented vegetables, legumes, and cereals exhibit significant differences from animal-based products. Within plant-based fermented foods, we observed differences between cereal-based, legume-based, and vegetable-based ferments. Similarly, there were variations between fermented dairy and fermented animal products (seafood and meat).
Traditional fermented foods serve as natural reservoirs of diverse bacteria and fungi
To assess whether microorganisms in fermented foods permeate food surfaces, we aliquoted 64 samples of Nepali fermented foods into duplicate tubes before conducting DNA extractions. One aliquot of each fermented food sample underwent rigorous vortexing to dislodge surface microbiota, while the replicate samples were homogenized using a mortar and pestle to capture all microbes associated with the surface through the core of food samples. Microbial communities obtained from fermented food samples from both approaches differed significantly from the extraction controls (Supplementary Fig. 1). But we observed no significant differences in the diversity, composition, and relative abundances of bacterial or fungal amplicon sequence variants (ASVs) between the homogenized and non-homogenized fermented food samples (Supplementary Fig. 1), indicating that microbes on the surface of fermented foods are indistinguishable from those present in the cores. Thus, we merged the sequencing reads obtained from homogenized and non-homogenized fermented food samples for subsequent analyses.
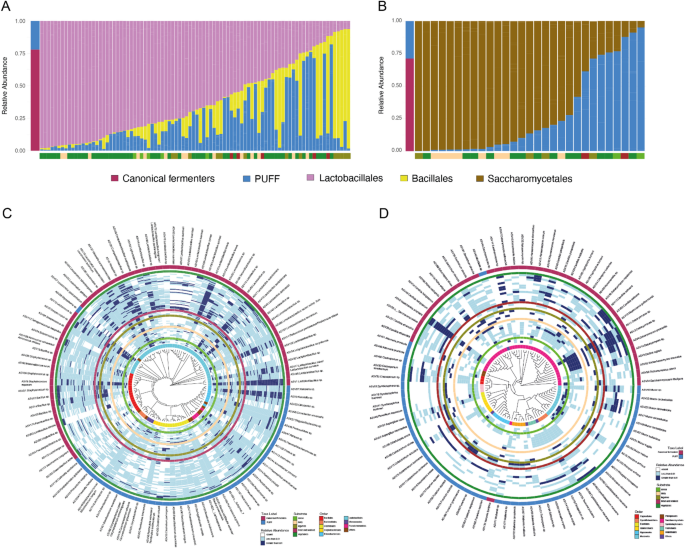
In total, we analyzed the bacterial communities in 90 fermented food samples using 16 S rRNA sequencing. Additionally, we examined fungal diversity in 29 samples that yielded sufficiently high DNA concentrations for ITS2 sequencing (Supplementary Fig. 1). Across the dataset, we identified a total of 104 bacterial and 91 fungal amplicon sequence variants (ASVs) exceeding relative abundance of 0.1%. Among the bacterial ASVs, 46 belonged to the order LABs, while 16 were members of the Bacillales order. Together, the LABs and Bacillales accounted for 78% of total bacterial reads (Fig. 2a). Likewise, Saccharomycetales emerged as the predominant fungal order in these fermented food samples, with less than half ASVs (40 of 91) comprising 71% of total fungal reads (Fig. 2b). We classified these microbial taxa as “Canonical Fermenters” due to their well-established role in food fermentation [45,46,47,48]. On average, each sample harbored 35 (SD ± 4), 11 (SD ± 2), and 18 (SD ± 7) ASVs from LABs, Bacillales, and Saccharomycetales, respectively (Supplementary Fig. 2a). While LABs, Bacillales, and Saccharomycetales were present in all samples, their relative abundances varied significantly within each food type (Supplementary Fig. 2b, p = 0.0002, 0.002, 0.004 respectively, Brown-Forsythe test). Fermented vegetables, dairy products, and alcoholic beverages were rich in LABs and Saccharomycetales, while fermented legumes, meats, and seafood showed elevated levels of Bacillales (Fig. 2c-d, Supplementary Fig. 2b). Lactiplantibacillus sp., a bacterial genera widely known for food fermentation [48], had the highest prevalence in our dataset and was found across all five substrate types (Fig. 2c). Pediococcus pentosaceus/Pediococcus stilesii, known to produce sour flavor [12], was more prevalent in fermented vegetables and cereal, which are desirable qualities in these foods. Issatchenkia orientalis, known to tolerate highly acidic conditions and previously extracted from a variety of fermented foods [49, 50], was the most prevalent fungi in our data. On the other hand, several members of yeasts from genera Debaryomyces dominated fermented dairy (Fig. 2d).
Traditional fermented foods exhibit heterogeneity in their microbial composition. A Leftmost column shows the relative abundances of Canonical Fermenters (Lactobacillales and Bacillales) across all samples in maroon and bacteria whose roles are Previously Undefined in Food Fermentation (PUFF) in blue. Subsequent columns show the relative abundances of Lactobacillales (pink), Bacillales (yellow), and PUFF bacteria (blue) in each sample. The bottom bar indicates substrate type: vegetables (darkgreen), cereals (light green), legumes (olive), dairy (beige), and animal products (brown). B Leftmost column shows the relative abundances of Canonical Fermenters (Saccharomycetales) across all samples in maroon and PUFF fungi in blue. Subsequent columns show their relative abundances in each sample. The bottom bar indicates substrate type colored as described above. C-D Phylogenetic tree of the bacterial and fungal ASVs and their relative abundances across all samples. The innermost ring represents the taxonomic Order. Subsequent rings represent individual samples grouped under the five colored rings corresponding to the respective substrate types. The outermost rings indicate whether a specific ASV is classified as Canonical Fermenter (maroon) or PUFF (blue)
In addition to the canonical fermenters, a notable portion of both bacterial (22%) and fungal (29%) reads represented microbes whose roles are previously undefined in food fermentation (PUFF, Figs. 2c-d). The bacterial and fungal ASVs representing PUFF were distributed across our samples originating from broad geographic regions (Fig. 2c-d), consistent with their detection in diverse traditional fermented foods in previous studies [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. Among the notable bacteria in this category are members of the genera Brevibacterium, Corynebacterium, and Salinivibrio. Brevibacterium and Corynebacterium were found at higher relative abundances in our cheese samples and they are known to produce red or orange colors in smear-ripened cheeses [31]. Salinivibrio, previously isolated from fermented seafood and soy products [32, 33], was most abundant in the fermented shrimp and soy sauce samples we obtained from South Korea (Supplementary Fig. 2c). Prominent PUFF fungi include ethanol-producing Rhizopus arrhizus and Mucor indicus [43]. These fungi were most prevalent in chhyang, the Nepali cereal based alcoholic beverage and its amylolytic starter, marcha. Likewise, Tausonia pullulans, primarily reported in sauerkraut and kimchi [34, 35], exhibited highest relative abundances in our vegetable samples. The fungus Fusarium oxysporum, which thrives on free amino acids in fermented meat products [51], was most abundant in our fermented meat samples (Supplementary Fig. 2d). Approximately 10% of bacterial and fungal taxa in our dataset belonged to plant endophytes, human skin commensals, and soil inhabitants. A key microbe in this category includes Pseudomonas, a soil-dwelling bacterium and common plant endophyte [52], which was detected in all traditional fermented foods in this study.
Abiotic factors shape the microbial compositions of traditional fermented foods
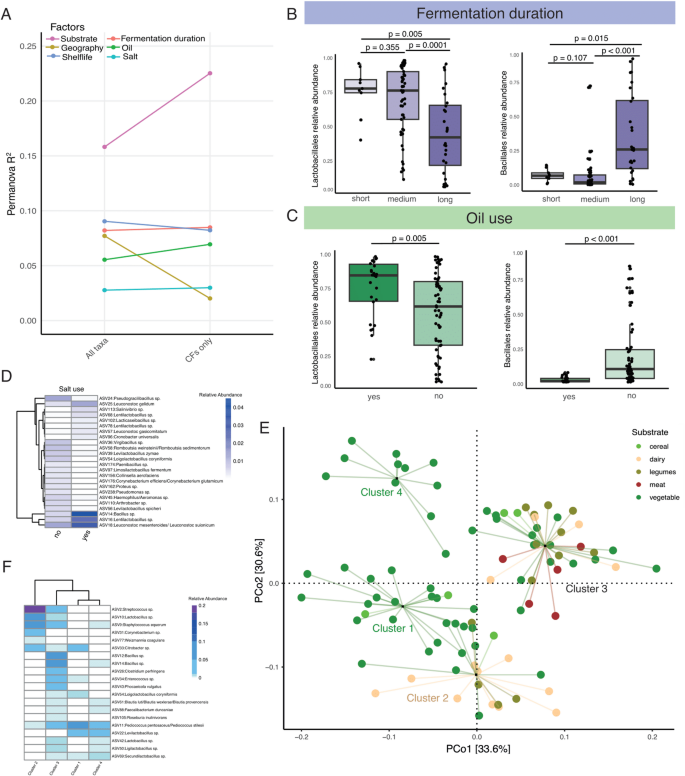
To identify factors contributing to the heterogeneity of microbial communities in diverse fermented foods, we conducted Principal Coordinate Analysis (PCoA) using weighted UniFrac dissimilarity distances of bacterial ASVs, followed by PERMANOVA with 10 variables associated with preparation methods (Fig. 3, Supplementary Fig. 3). When analyzing all 104 bacterial ASVs, we detected a significant association between geography and bacterial community structure (Fig. 3a, p = 0.0001, PERMANOVA). However, repeating these analyses after removing the ASVs classified as PUFF resulted in marked reduction in the effect of geography on bacterial composition of these fermented foods (Fig. 3a, p = 0.38, PERMANOVA). These results indicate that geographical differences in fermented foods emerge from bacteria whose contributions in fermentation processes are not fully understood.
Among the 10 variables included in the PERMANOVA, the substrate type exhibited the largest effect on the bacterial community structure of these fermented foods (Fig. 3a, p , PERMANOVA). Unlike geography, the effect of food substrate on the bacterial composition of traditional fermented foods was more pronounced when the PUFF microbes were excluded. This indicates that the nutrients provided by the food substrates are the primary determinants of bacteria in traditional fermented foods.
The bacterial compositions of traditional fermented foods are shaped by substrate type, preparation methods, and shelf life. A PERMANOVA on the weighted UniFrac dissimilarity matrix identifies factors significantly associated with bacterial composition in traditional fermented foods. B The abundances of LABs decrease while that of Bacillales increase with longer fermentation periods. C Foods prepared without oil show an increase in the relative abundances of Bacillales and a decrease in LABs. D Differentially abundant ASVs between salted and unsalted fermented foods. E Principal Coordinate Analysis (PCoA) on the weighted unifrac dissimilarity matrix of traditional fermented food samples using 16 S rRNA reads. Circles representing samples are grouped using PAM clustering and lines connect each sample to the cluster centroid. Colors represent substrate types: vegetables (darkgreen), cereals (light green), legumes (olive), dairy (beige), and animal products (brown). F Relative abundances of top 20 taxa with the highest variable importance factor (VIF) in the random forest model used to distinguish the four clusters of food samples
In addition to substrate and geography, fermentation duration, the use of oil and salt, and shelf-life were also significantly associated with bacterial composition (Fig. 3a, p , PERMANOVA). Foods fermented for longer periods and not immersed in oil constituted lower relative abundances of LABs and higher proportions of Bacillales (Fig. 3b-c, p , Mann Whitney test). Similarly, we identified 24 bacterial ASVs that were differentially abundant between salted and unsalted foods (Fig. 3d, FDR adjusted p 2, MaAsLin2). Among these, Salinivibrio sp. and several members of Bacillus, Lacticaseibacillus, Lentilactobacillus, and Leuconostoc were more prevalent in salty foods. This finding is consistent with earlier studies that identified Salinivibrio and Leuconostoc mesenteroides as capable of thriving in high-salt environments [53, 54].
Next, we performed partition-around-medoids (PAM) clustering using the first four axes of the bacterial Principal Coordinate Analysis (PCoA), which revealed that the 90 samples of diverse fermented foods acquired from various geographical locations segregated into four broad clusters (Fig. 3e, Supplementary Fig. 3). The first cluster predominantly comprised of spontaneously fermented fiber-rich vegetables and cereals from Nepal (p = 3.74e-05, Fisher’s exact test). The second cluster was enriched in dairy products (p = 6.23e-05, Fisher’s exact test), while protein-rich legumes and animal products from Nepal and South Korea were overrepresented in the third cluster (p = 3.82e-04, Fisher’s exact test). Notably, all fermented vegetables exceeding one year of shelf life were detected in the fourth cluster and showed higher relative abundances of bacteria associated with sour taste such as Levilactobacillus brevis and Lactobacillus acetotolerans [13,14,15], suggesting bacterial communities of fermented foods change over time. Notably, Leviactobacillus brevis is known to withstand low pH and has previously been reported in over ripened kimchi [15].
To validate these clusters independently, we employed a random forest classifier, which distinguished these four clusters with 100% accuracy (Supplementary Fig. 3). Furthermore, the bacteria with the highest variable importance factor (VIF) scores in the random forest classifier that were crucial for distinguishing the four clusters exhibited substrate specificity (Fig. 3f). For example, Streptococcus sp., Corynebacterium sp., Brevibacterium sp., and Weizmannia coagulans, previously associated with fermented dairy products [31, 55, 56], distinguished Cluster 2, which was enriched for the dairy samples. Likewise, Bacillus sp. prominent in soybean fermentation [57], delineated Cluster 3 comprising legumes and animal products. Finally, several LABs, known for their importance in vegetable fermentation [58], were prevalent in both Clusters 1 and 4 that were enriched for fermented vegetables. These results collectively indicate that despite differences in geographical origins and variations in the fermentation processes, substrate type has a significant contribution in shaping the bacterial composition of fermented foods.
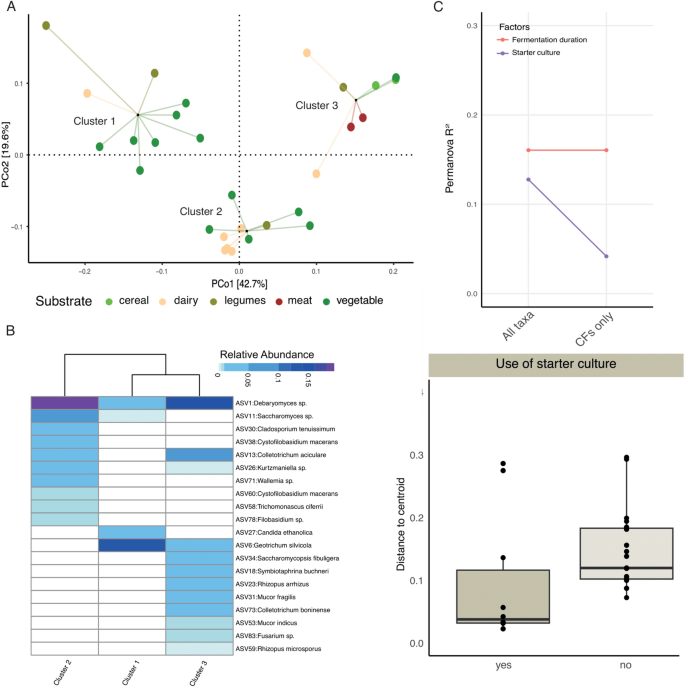
When these analyses were conducted using the fungal ASVs, neither geography nor substrate type showed significant associations with the fungal compositions of these fermented foods (Supplementary Fig. 4, p PERMANOVA). Clustering analyses revealed these fermented foods can be grouped into three clusters, which was verified by a random forest classifier (Fig. 4a, Supplementary Fig. 4), but these clusters were not linked to substrate types (p > 0.05, Fisher’s exact test). The fungal species with the highest VIF scores (Fig. 4b) also did not show substrate specificity (Supplementary Fig. 4). Instead, fermentation duration and the use of starter cultures appeared to be the major determinants of the fungal communities in these fermented foods (Fig. 4c). A dispersion analysis revealed that fungal communities in foods fermented using starter cultures showed greater uniformity relative to the spontaneously fermented foods (Fig. 4d, p Mann Whitney test). To determine whether the lack of association of substrate and geography with the fungal community structure was due to a smaller sample size of the fungal dataset, we analyzed the bacterial ASVs data from the same 29 samples. Despite the reduced sample size, bacterial composition remained significantly associated with substrates (Supplementary Fig. 5), indicating that different abiotic factors contribute to bacterial and fungal diversity in traditional fermented foods.
The fungal compositions of traditional fermented foods are shaped by fermentation duration and use of starter cultures. A Principal Coordinate Analysis (PCoA) of the weighted UniFrac dissimilarity matrix of traditional fermented foods using ITS2 reads followed by PAM clustering. Samples are represented by circles color coded by substrates and linked to the cluster centroids by lines. B Relative abundances of top 20 taxa with the highest VIF in the random forest model used to distinguish three food clusters. C PERMANOVA on the weighted UniFrac dissimilarity matrix identified factors significantly associated with the fungal composition. D Dispersion analysis using weighted UniFrac distances comparing spontaneously fermented foods with those fermented using starter cultures
Bacterial pathways differ by fermented food substrates
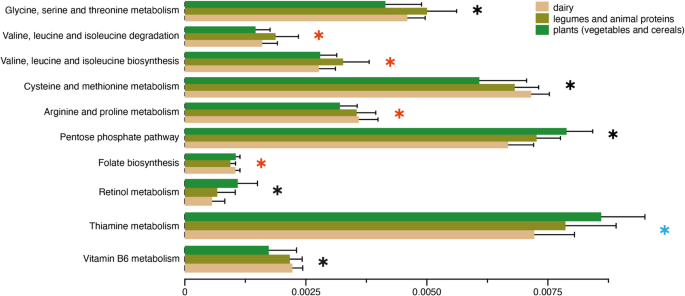
Considering the significant role of substrate in shaping bacterial communities in traditional fermented foods, we sought to examine whether the bacterial communities in different substrates also differ functionally. Using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt2) [59], which enables prediction of functional content of microbial communities from amplicon sequencing data, we inferred the relative abundances of Kyoto Encyclopedia of Genes and Genomes (KEGG) [60] pathways in our fermented foods. Comparison of KEGG pathway relative abundances revealed distinct functional profiles across different substrates (FDR adjusted p 5, Supplementary Tables 3,4). Bacteria in vegetable- and cereal-based fermented foods were enriched for several pathways involved in carbohydrate metabolism (PPP), folate biosynthesis, as well as retinol (Vitamin A) and thiamine (Vitamin B1) metabolism. Plants contain high fibers, which provides the ideal substrate for bacteria that metabolize carbohydrates. Folate, an essential micronutrient necessary for cell growth and red blood cell formation [61], is often recommended during pregnancy to reduce the risk of neural tube defects in infants [62]. Retinol is important for vision, cellular development, and immunity [63], while thiamine is vital for glucose metabolism, as well as nerve, muscle and heart function [64]. Similarly, compared to plant based fermented foods, bacterial communities in fermented dairy, legumes, and animal products showed higher abundances of pathways involved in amino acid metabolism consistent with their macronutrient profiles. They were also enriched for Vitamin B6 metabolism, which is vital for nervous and immune system functions [65]. While these findings underscore the functional potential of microbes in traditional fermented foods, future shotgun metagenomic studies could provide deeper insights into their metabolic capabilities. Such studies may reveal microbial functions that correspond to the macronutrient composition of food substrates and identify biosynthetic compounds with potential benefits for human health.
Bacterial functions may differ across food substrates. Relative abundances of putative functional pathways that are significantly different among substrate types (LinDA, p adjusted ). Pathways with a blue star are significantly different between plants and dairy, those with a red star are significantly different between plants versus dairy, legumes and animal products, and those with a black star are significantly different across all three substrate types
Biotic interactions influence fermented food microbial communities
Microbial interactions play a pivotal role in shaping the microbial communities in fermented foods, consequently influencing their community structure and functions. Some microbes act as primary decomposers, breaking down food substrates to supply nutrients and metabolites to neighboring microbes, thereby facilitating their growth [66]. Others produce toxins that inhibit the proliferation of nearby microbial cells, shaping the overall community composition [67]. To elucidate the microbial interactions in traditional fermented foods, we constructed co-occurrence networks using 104 bacterial ASVs from all 90 food samples and 91 fungal ASVs from the 29 ferments in our dataset. Our analyses revealed densely interconnected bacteria-bacteria and fungi-fungi networks within these fermented foods (Supplementary Fig. 6). We identified five distinct co-abundance groups (CAGs) for both bacteria and fungi that varied in connectedness and transitivity. Connectedness measures the number of edges (co-occurrences) of each node (ASV) within a CAG, normalized by the total number of possible edges of nodes in the CAG. Transitivity assesses how often two interconnected nodes co-occur with a common node. (Supplementary Fig. 7). Both metrics evaluate how densely ASVs are interconnected within each CAG. We also identified focal microbes within each CAG by assessing their centrality using three measures: strength, betweenness, and eigenvector centrality (Supplementary Fig. 8). Microbes with high centrality are indicative of keystone species in microbial ecosystems [68].
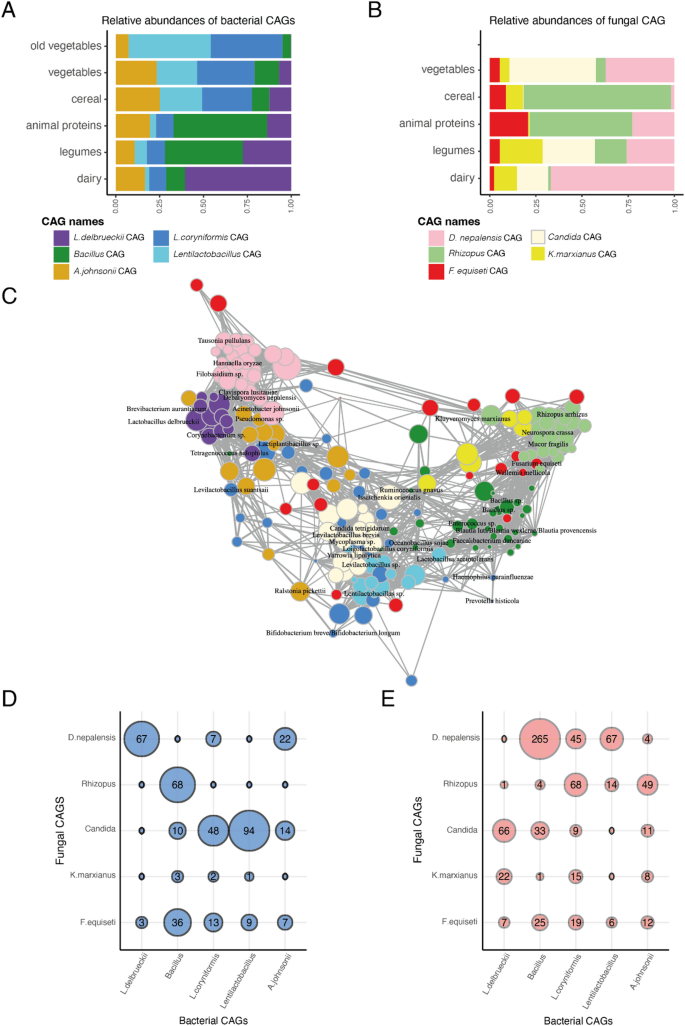
Among the bacterial CAGs, the Lactobacillus delbrueckii-CAG emerged as the most interconnected and showed highest transitivity. It consisted of Lactobacillus delbrueckii and two PUFFs, Brevibacterium sp. and Corynebacterium sp. as focal members of this CAG. The L. delbrueckii-CAG was predominantly found in dairy products within our dataset (Fig. 6a), consistent with association of these bacteria with cheesemaking processes [31, 69]. A second bacterial CAG that was prominent in legumes, meat, and seafood in our dataset comprised members of Bacillus as the focal taxa along with Tetragenococcus halophilus, Oceanobacillus sojae, Enterococcus sp., and Salinivibrio sp, which is consistent with the roles of these bacteria in soy and protein fermentation [33, 47]. In addition, several human gut associated bacteria that were categorized as PUFF, including Faecalibacterium, Blautia, and Ruminococcus gnavus, showed high centrality measures in this Bacillus-CAG. Conversely, the fermented cereals and vegetables in our dataset were distinguished by two bacterial CAGs that demonstrated high connectedness. One of them comprised Loigolactobacillus coryniformis and Levilactobacillus suantsaii as the focal species but it also consisted of other canonical fermenters such as Leuconostoc mesenteroides/suionicum, Lactiplantibacullis sp. as well as PUFF bacteria such as Arthrobacter, Proteus and human gut microbiome associated bacteria such as Prevotella histicola and Bifidobacterium. The second CAG, consisted of Lactobacillus acetotolerans as a focal species. L. acetotolerans is associated with sour taste in vinegar, pickles, and alcoholic beverages [13, 70] and these are the desired traits in the fermented vegetables from Nepal and South Korea. The fermented vegetables with longer shelf lives had a markedly higher proportion of this CAG. Finally, the fifth bacterial CAG consisted of several bacteria commonly associated with plant endophytes and soil such as Acinetobacter johnsonii, Ralstonia picketti, and Pseudomonas sp. as central members. This CAG did not show substrate specificity and was present at low proportions across all substrates.
Co-occurrence analysis reveals densely interconnected microbial ecosystems in traditional fermented foods. A, B Relative abundances of bacterial and fungal co-abundant groups (CAGs) across substrate types. C Bacteria and fungi that significantly co-occur with one another in traditional foods. D, E The number of significant positive and negative associations between bacterial and fungal CAGs
In addition to the positive interactions between bacteria within each CAG, we found antagonism between the five bacterial CAGs. The L. delbrueckii-CAG that defined the dairy bacterial community was highly antagonistic to the Bacillus-CAG prominent in legumes, meat, and seafood. Similarly, bacterial members of the two CAGs consisting of LABs and prevalent in vegetables showed several negative interactions with both the L. delbrueckii-CAG and the Bacillus-CAG.
Unlike bacteria, most of the fungal CAGs displayed a broader substrate distribution, with three prominent CAGs consisting of Debaryomyces nepalensis, Candida, and Rhizopus as focal species, respectively (Fig. 6b). These CAGs demonstrated high connectivity and transitivity and were characterized by distinct focal species from the PUFF category that potentially play key roles within the microbial network. Some key focal members of the D. nepalensis-CAG consisted of several yeasts including Tausonia pullulans, Clavispora lusitaniae, Filobasidium sp., and the umami-associated Hannaella oryzae [21]. The Candida-CAG consisted exclusively of yeasts including low pH tolerant Yarrowia lipolytica and Issatchenkia orientalis that are associated with appearance, flavor, and taste of diverse fermented foods [49, 50, 71]. The Rhizopus-CAG included molds such as Neurospora crassa known to degrade allergens in soybean [72] and Mucor fragilis known for producing bioactive compounds [73]. The fourth CAG consisted of flavor-enhancing fermentation capable yeast, Kluyveromyces marxianus as a central species [18, 74]. The final fungal CAG consisted of several environmental fungi including Fusarium equiseti and Wallemia mellicola. The D. nepalensis-CAG was highest in dairy (~ 67%) but also detected at high proportions in vegetables (37%). The Rhizopus-CAG was high in fermented cereals and meat samples, while the Candida-CAG was detected at appreciable proportions in vegetables, legumes, and dairy. The remaining two fungal CAGs were distributed across the different substrates at low proportions.
Moreover, joint co-occurrence network analysis of bacterial and fungal ASVs revealed extensive symbiotic and antagonistic relationships across the two kingdoms, while maintaining the within-kingdom network architecture described above (Fig. 6c-e). Notably, bacterial members of the L. delbrueckii-CAG that was highly prevalent in dairy frequently co-occurred with the fungi in the D. nepalensis-CAG and showed negative interactions with the Candida-CAG. In contrast, the Bacillus-CAG was highly antagonistic to D. nepalensis-CAG but co-occurred with members of Rhizopus-CAG. Furthermore, members of the two LAB dominated CAGs associated with fermented vegetables coexisted with fungi in the Candida-CAGs, which consisted of several acid tolerant species, indicating that both bacteria and fungi may contribute to create a low pH environment where specific bacteria and fungi can proliferate. Overall, our findings highlight the complex dynamics of microbes within fermented food communities and underscore their intricate interdependence that shape the microbial ecosystems in traditional fermented foods.