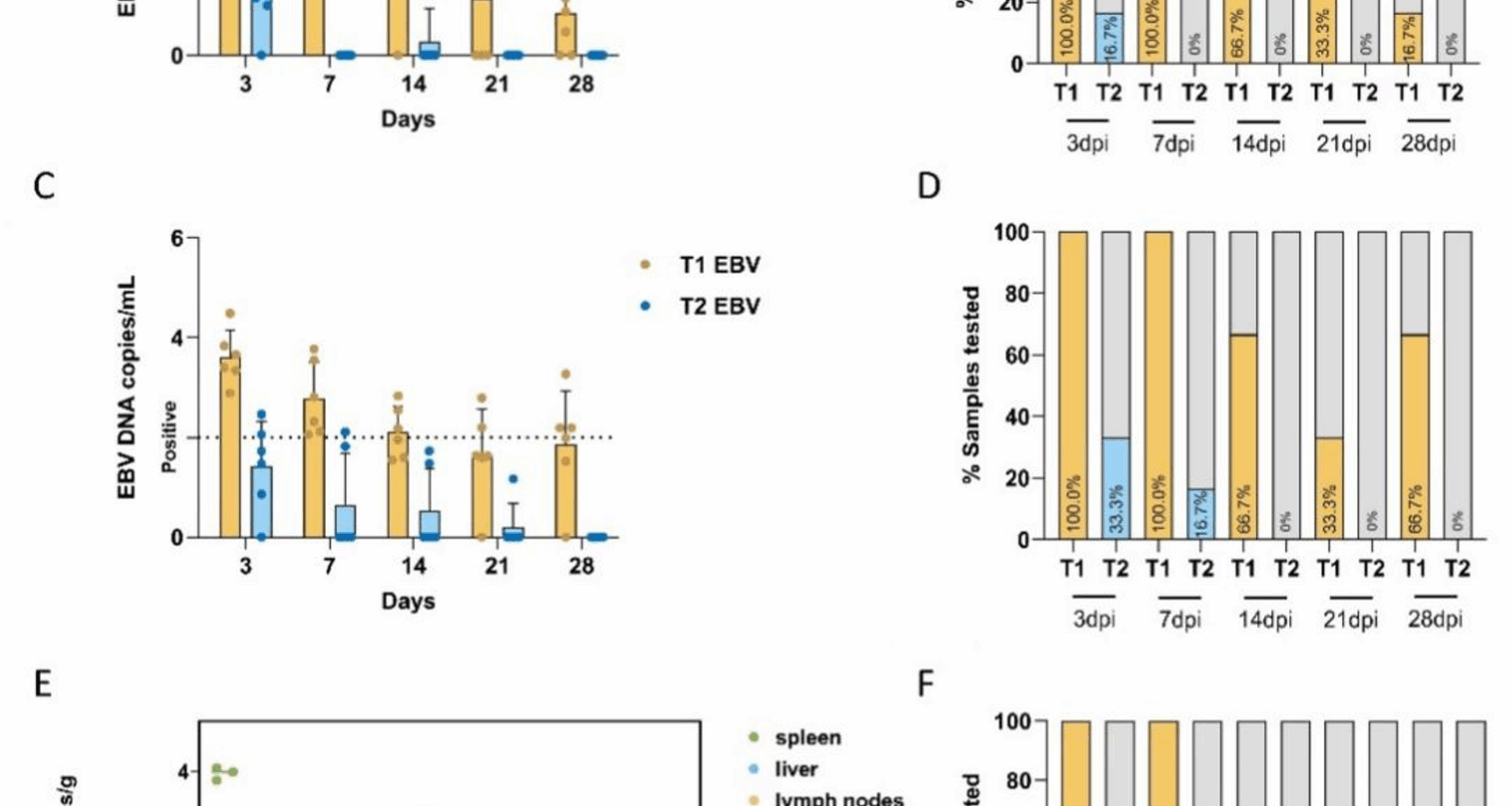
The mechanisms of infection and pathogenesis associated with EBV remain poorly understood. The pronounced species specificity of EBV is a major obstacle to in vivo investigations [21]. Our previous study established that type-1 EBV (derived from B95-8 cells) infects tree shrews (Tupaia belangeri), producing persistent viral carriage in the absence of overt clinical signs, thereby positioning the tree shrew as a candidate small animal model for EBV infection [16]. In this study, we compared the in vivo infection efficiencies and characteristics of different EBV subtypes. We found that EBV-DNA was only transiently detected in the PBMC of animals (T2-1), but EBERs-ISH-positive cells were not found in any tissue. These findings imply that T2 EBV may establish a short-lived infection that is eliminated by innate and/or adaptive immune responses. In contrast, T1 EBV showed higher viral loads in PBMC three days post-inoculation, with a 100% detection rate within one week and persistence for at least 28 days. The EBV DNA load and detection rate in the tissues showed significantly greater viral DNA burdens and positivity rates in the spleen and lymph nodes of type-1-infected shrews than in their type-2 counterparts. The positivity rate of the three T1 group tree shrews detected was 100%, suggesting that these tissues may be the main latent and replication sites for EBV. Consistently, LMP1 and EBER transcripts were readily detected in these tissues, supporting their roles as the principal sites of latent infection and viral replication. Conversely, type-2 EBV yielded no detectable LMP1 or EBER signals, underscoring its reduced infectivity and inability to establish latency. The detection rates in PBMC and key immune organs suggest that T2 EBV has a lower infection efficiency in tree shrews than T1 EBV. Similar to a rabbit model study where P3HR-1 EBV showed a lower infection rate (17%) compared to B95-8 EBV (91%) in rabbits [22]. Notably, tree-shrew complement receptor 2 (CR2), the primary EBV entry receptor in B cells, shares 100% identity at critical residues with the human orthologue, far exceeding the 50% identity observed in rabbits [16]. The literature indicates that the rabbit model has differences in EBV antibody titers compared to human responses [19] and shows the absence of EBNA1 protein in infected rabbits [23]. Collectively, these findings suggest that tree shrews may be a more suitable animal model than rabbits for EBV infection research. Although humanized mouse models can show EBNA2-deleted EBV isolates, P3HR-1 causes Hodgkin-like lymphomas and diffuse large B cell lymphomas with T2 and Wp-restricted latency types in humanized mice; however, they have limitations such as incomplete immune microenvironments and an inability to replicate the natural EBV infection process. Therefore, the development of small animal models that more closely mimic human EBV infection remains an urgent priority. Comprehensive in vitro and in vivo analyses are required to determine how EBV T1 and T2 differentially evade immunity, maintain latency, and drive oncogenesis, thereby identifying novel therapeutic targets.
One possible explanation for the reduced infection efficiency of tree shrews inoculated with T2 EBV is that P3HR-1 EBV lacks EBNA2, and its characteristics may not fully represent all T2 EBV. In contrast, B95-8 EBV, which retains intact EBNA2, is more competent at sustaining latency and promoting cellular proliferation in vivo [24, 25]. In our RNA-seq analysis, we identified NR4A1 (log2FoldChange = 1.9, padj 26, 27]. Marked differences in T-cell immune responses have been observed between T1 and T2 EBV infections, which may help explain the distinct biological behaviors of these two subtypes. In addition, some studies have reported that EBNA2 exerts anti-apoptotic functions through its interaction with NR4A1, thereby providing survival advantages to infected cells [28]. Based on these findings, we hypothesize that during latent infection with type 1 EBV, the synergistic effect of EBNA2 and NR4A1 may contribute to the regulation of cell survival. In contrast, in type 2 EBV infection, the absence of EBNA2 may lead to stronger T-cell–mediated cytotoxicity and enhanced pro-apoptotic activity, which could be an important reason why type 2 EBV struggles to establish persistent infection. This hypothesis offers a novel perspective for explaining the subtype-specific differences in the capacity of EBV to sustain infection. Compared with T1 EBV infection, although T2 EBV displays a lower infection efficiency against tree shrews, the model remains useful for characterizing the in vivo behavior of EBNA2-deficient viruses. The rapid clearance of T2 EBV in vivo may trigger a distinct host immune cascade that could inform future prophylactic and therapeutic interventions.
This study provides a new animal model for comparing T1 and T2 EBV infections in vitro. However, there are some limitations that need to be addressed. First, EBV derived from P3HR-1 lacks the EBNA2 gene and may not fully represent all types 2 EBV. Using additional type 2 EBV strains for multiple verifications can more comprehensively assess the characteristics and behaviors of EBV2 types and compensate for the possible limitations of a single cell line, thereby increasing the reliability and universality of the research results. Future investigations will need to incorporate additional T2 EBV cell lines, such as AG876, to rigorously validate these findings and provide a more comprehensive understanding of T1–T2 EBV differences. In addition, because of the reproductive cycle and cost constraints of the experimental animals, the sample size of tree shrews included in this study was relatively limited, and larger cohorts will be required to increase the statistical power. Secondly, because tree shrews are not conventional laboratory animals, species-specific serological reagents are not available. Therefore, it is necessary to develop specific detection tools for viral infections in tree shrews. In addition, the study relied solely on in vivo data and lacked verification of the EBV infection mechanism at the cellular level in vitro. Complementary in vitro experiments, for example, with cultured tree-shrew peripheral PBMCs, are necessary to delineate the viral entry, latency, and immune evasion mechanisms. Finally, although differences in infection between type 1 and type 2 EBV in tree shrews were observed, the molecular determinants underlying the divergent latent programs and immune escape strategies of EBV types 1 and 2 remain to be elucidated. Addressing these issues will refine the tree-shrew model, including optimizing animal models, conducting comprehensive monitoring, and exploring the infection characteristics of EBV, which will advance our understanding of EBV pathogenesis.