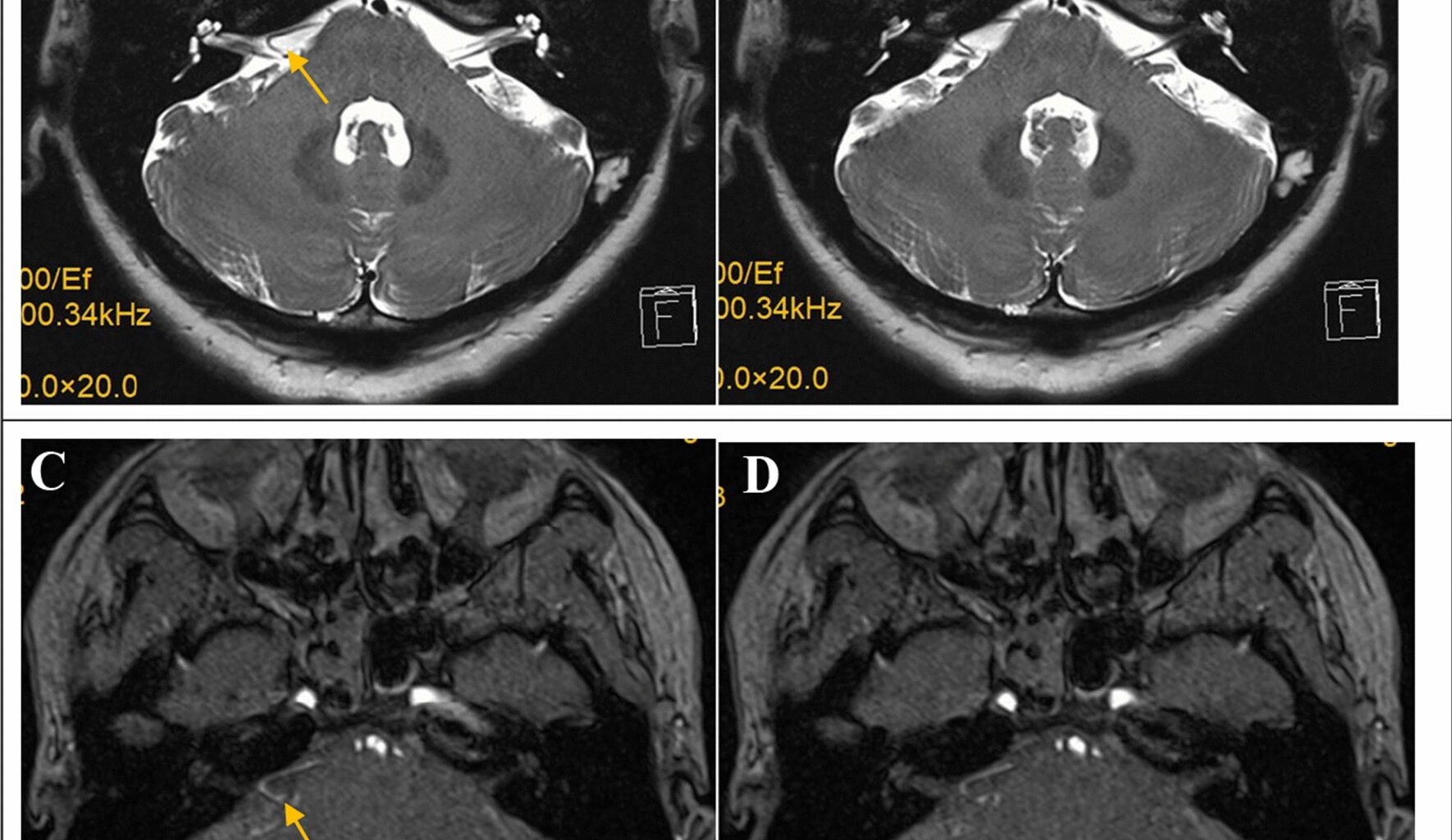
HFS is a common functional disease treated by neurosurgery, as it is a type of cranial nerve disease. The incidence rate is approximately 0.78/100000 people, although this rate among women is twice that among men [13]. HFS should be distinguished from diseases, such as Meige syndrome, habitual facial spasms, and sequelae of facial paralysis. Meige syndrome is often characterized by bilateral blepharospasm, which gradually develops downward and may be accompanied by involuntary movements of the mouth, jaw, and neck muscles. Habitual facial muscle twitching is mostly bilateral, and patients can control their twitching movements independently without facial sensory disorders. The sequelae of facial paralysis may manifest as coordinated movements and spasms of facial muscles, but there is a history of facial paralysis. Although it is not a fatal disease, it can exert significant psychological pressure on patients. Currently, the REZ of the facial nerve is compressed by vessels, resulting in changes in nerve demyelination, short circuits of nerve conduction, and increased nerve excitability [14]. Although the vast majority of HFS cases are caused by compression of the root of the facial nerve, with advancements in medical technology, especially neurophysiological monitoring technology, it has been discovered that not all HFS cases are caused by compression of the REZ of the facial nerve. It has been reported that compression of the cisternal portion of the facial nerve may also be the cause of HFS, or it could be a combination of both. According to reports, 94.6% of cases are due to REZ compression, 0.7% are due to simple distal compression, and 4.7% are due to mixed compression [15]. Scholars have reported HFS cases caused by distal compression of the facial nerve [16, 17], and many of them have explored not only the REZ but also the cisternal portion of the facial nerve during MVD. The most common compression site for HFS caused by distal compression of the facial nerve is in the middle of the brainstem segment. Although some reports mention that the compression site is close to the IAC [18, 19], it is still in the brainstem segment. However, vascular compression in the IAC is very rare and is easily overlooked during surgery. The most common vessel responsible for HFS is the AICA, followed by the vertebral artery and posterior inferior cerebellar artery [20]. The case presented in our study is rare, as the vessels near the brainstem and facial nerve roots were not responsible. The responsible vessel was the LA, which was hidden in the IAC. Furthermore, the compression location was not at the root or brainstem; it was in the IAC. The LA of this patient was longer and more tortuous than usual, which may explain the LA compression. There were no obvious abnormalities in the IAC anatomy in this patient. This is extremely rare and has not been reported to date. The compression in previously reported HFS cases caused by distal compression of the facial nerve in the IAC was generally attributed to AICA loops or other space-occupying lesions. In this case, the patient experienced compression of the LA in the IAC, which was more concealed and easily overlooked. For patients with HFS caused by distal compression of the facial nerve, we should be aware of the possible existence of compression in the IAC during surgery and explore the IAC. Given the elongated course and limited mobility of the LA, decompression of the facial nerve in the IAC requires meticulous manipulation to avoid complications such as facial paralysis and hearing loss from injuries to the LA and its branches. Postoperative nimodipine is essential to prevent neuroischemic complications from LA vasospasm. This study provides a new understanding of the causes of HFS and provides new guidance for the surgical treatment of refractory HFS.
Early-stage HFS can be treated with medication, but as the disease progresses, most patients ultimately require surgical treatment. Currently, the most commonly used and effective surgical method is MVD. First proposed by Professor Jannata PJ in 1967, MVD has now become an internationally recognized standard method for the clinical treatment of cranial nerve disorders, such as HFS and trigeminal neuralgia [21]. The advantage of this surgery is that it can relieve local vascular compression and eliminate symptoms while preserving the normal function of the facial nerve [22]. The key to the success of MVD lies in the separation and isolation of the responsible vessels that compress the facial nerve during the operation. In the past, the determination of the offending vessels in MVD mainly relied on the clinical experience of the surgeon, which inevitably led to the possibility of missing hidden offending vessels, especially in rare cases, such as HFS caused by compression of the facial nerve in the IAC, which is generally difficult to detect without neuroelectrophysiological monitoring. With the continuous development of medical technology and equipment, neuroelectrophysiological monitoring technology has been applied in MVD, which can objectively guide the identification of offender of compression and allow for real-time judgment of the surgical effect during surgery, guiding the treatment of patients with HFS [23]. Neuroelectrophysiological monitoring technology has made the well-established and conventional MVD more effective and safer [24].
The AMR, also known as the lateral spread response (LSR), is the main neuroelectrophysiological monitoring tool used in MVD for HFS. The AMR is a characteristic electrophysiological manifestation of HFS, and the accuracy of monitoring the effect of MVD for HFS is very high [25]. The AMR can monitor the decompression effect in real time and can detect many special cases of HFS, including rare situations, such as those involving microvessels, venous vessels, and nonbrainstem blood vessels [6, 26]. The AMR usually involves dual branch monitoring of the facial nerve, including (1) stimulating the temporal branch of the facial nerve and recording it in the chin muscle and (2) stimulating the mandibular marginal branch and recording it in the orbicularis oculi muscle. Compared with single branch detection, dual branch detection has better sensitivity and accuracy. After dual branch monitoring indicated that the AMR had disappeared, the postoperative effectiveness of MVD in patients with HFS reached 98% [27, 28]. In this case, the patient also underwent dual branch monitoring with a current of 30 mA and 0.2 ms for stimulation. After decompression of the REZ of the facial nerve, the AMR from the orbicularis oculi muscle and the chin muscle did not disappear. Finally, with repeated exploration and monitoring of potential prompts, the offending vessels, namely, the LA and its branches located at the IAC, were found. Therefore, dual branch monitoring of the facial nerve of the AMR plays an important role in accurately and comprehensively identifying the offending vessels. Early detection of the IAC can accelerate the recognition of the LA. However, owing to the rarity of this case, we did not initially consider this factor and could only use neuroelectrophysiological monitoring to identify the offending vessels. Without neurophysiological monitoring results, the identification of the hidden offending vessels in the IAC would be difficult. Compared with typical cases involving the AICA, MVD was challenging in this case because compression of the facial nerve in the IAC was very rare and more concealed, making it difficult to identify offending vessels and prone to omission during surgery. Additionally, the LA and its branches are slender, exhibit less mobility, and are easily damaged, leading to hearing loss and facial paralysis.
On the basis of the analysis of this rare case, we summarize two lessons: (1) during MVD in HFS, neurophysiological monitoring of the AMR is essential to ensure that the offending vessels are identified, especially in rare cases. (2) If the AMR does not completely disappear after decompression of the REZ of the facial nerve, the facial nerve needs to be examined throughout the entire intracranial segment, including the REZ, the cisternal portion, and the IAC. All vessels that are closely related to the facial nerve should be separated until the AMR completely disappears.