Case 1: CAS combined with DLBCL
We report a case of a 29-year-old Han male patient. On 17 October 2024, he presented to the First Affiliated Hospital of Anhui Medical University with complaints of fatigue and palpitations for 2 weeks. A complete blood count revealed: white blood cell count of 4.12 × 109/L, hemoglobin of 76 g/L, platelet count of 315 × 109/L, and reticulocyte percentage of 15.26%. Biochemical tests showed: total bilirubin of 55.6 µmol/L, direct bilirubin of 18 µmol/L, indirect bilirubin of 37.6 µmol/L, and lactate dehydrogenase of 835 U/L. Tests for iron metabolism, urinalysis, and a panel of 10 autoantibodies showed no abnormalities. Both the direct antiglobulin test and blood typing yielded indeterminate results. The patient was diagnosed with hemolytic anemia and treated with oral prednisone acetate at a dose of 15 mg (three times daily). He was subsequently monitored with intermittent blood tests, and the steroid dosage was gradually tapered. On 8 February 2025, the patient returned to our hospital due to worsening fatigue and palpitations, along with chest tightness and shortness of breath, which intensified upon exertion.
Physical examination
The patient was alert and oriented, exhibited signs of severe anemia, and had mild scleral icterus. No enlargement of superficial lymph nodes was detected. Cardiopulmonary auscultation revealed no abnormalities. The liver and spleen were not palpable below the costal margin. No edema of the lower limbs or neurological abnormalities were observed.
Laboratory examinations
Complete blood count: white blood cell count 12.12 × 109/L, red blood cell count of 1.55 × 1012/L, hemoglobin of 58 g/L, platelet count of 497 × 109/L, reticulocyte percentage of 13.15%, and absolute reticulocyte count of 0.2038 × 1012/L. Liver function tests: total bilirubin of 99.1 µmol/L, direct bilirubin of 10.7 µmol/L, indirect bilirubin of 88.4 µmol/L, and lactate dehydrogenase (LDH) of 707 U/L. Immunoglobulin and complement panel: IgM of 5.02 g/L, complement C4 of 0.03 g/L. Cold agglutinin titer at 4 °C was 1:2048. Pretransfusion immunological screening showed blood type A, RhD-positive, with a positive irregular antibody screen. Coombs test: direct antiglobulin test (DAT) positive (C3d positive), indirect antiglobulin test (IAT) also positive. Serum immunofixation electrophoresis and serum protein electrophoresis revealed a monoclonal immunoglobulin of the IgM-K type with an M-protein band present; M-protein concentration was 3.26 g/L. Urine immunofixation electrophoresis and urine protein quantification showed a κ light chain precipitation band and M-protein band, with an M-protein level of 254.93 mg/24 hours. Serum free light chain analysis: serum free κ light chain 15.7 mg/L, serum free λ light chain 22.10 mg/L, with a κ/λ ratio of 0.71. Autoantibody panel (16 items): antinuclear antibody (ANA) positive, granular pattern at a titer of 1:100; all other items negative. No abnormalities were found in PNH clone detection, coagulation profile, urinalysis, respiratory pathogen panel (8 items), Epstein–Barr virus (EBV) DNA, cytomegalovirus (CMV) DNA, thyroid function panel (T3, T4, TSH), antiphospholipid antibodies, ANCA testing, β2-microglobulin, immunoglobulin subclass panel, thyroid antibodies, or fecal occult blood test. Bone marrow cytology: active proliferation of nucleated cells, granulocyte-to-erythroid ratio 0.51:1. Granulocytic lineage: cells at various stages displayed mostly normal morphology with some eosinophils observed. Erythroid lineage: elevated proportion of cells at all stages, with generally normal morphology of erythroblasts and anisocytosis among mature red blood cells. Lymphocyte proportion was decreased, with otherwise normal morphology. A total of 15 megakaryocytes were observed, including 5 platelet-producing megakaryocytes, 11 granular megakaryocytes, and 2 naked nuclei. Scattered platelets were also seen. Bone marrow immunophenotyping: no evidence of acute leukemia, high-risk myelodysplastic syndromes (MDS), lymphoma, or multiple myeloma-related immunophenotypic abnormalities. A significant relative increase in nucleated red blood cells was noted. Bone marrow biopsy: no increase in blasts or lymphocytes; few megakaryocytes were observed. Erythroid hyperplasia was mildly increased. The lab results were presented in Table 1.
Table 1 Lab results of case 1Imaging studies
Contrast-enhanced CT of the chest, abdomen, and pelvis revealed multiple enlarged lymph nodes in the retroperitoneum, mesenteric vessels, and bilateral iliac vascular regions, with the largest short axis measuring 30 mm. The spleen was of normal size, and no hepatic lesions were detected. Ultrasound of superficial lymph nodes showed lymphadenopathy in bilateral cervical and axillary regions. The left axillary lymph node (34 × 14 mm) exhibited abnormal structure, loss of the hilum, and abundant blood flow signals.
Pathology and immunohistochemistry
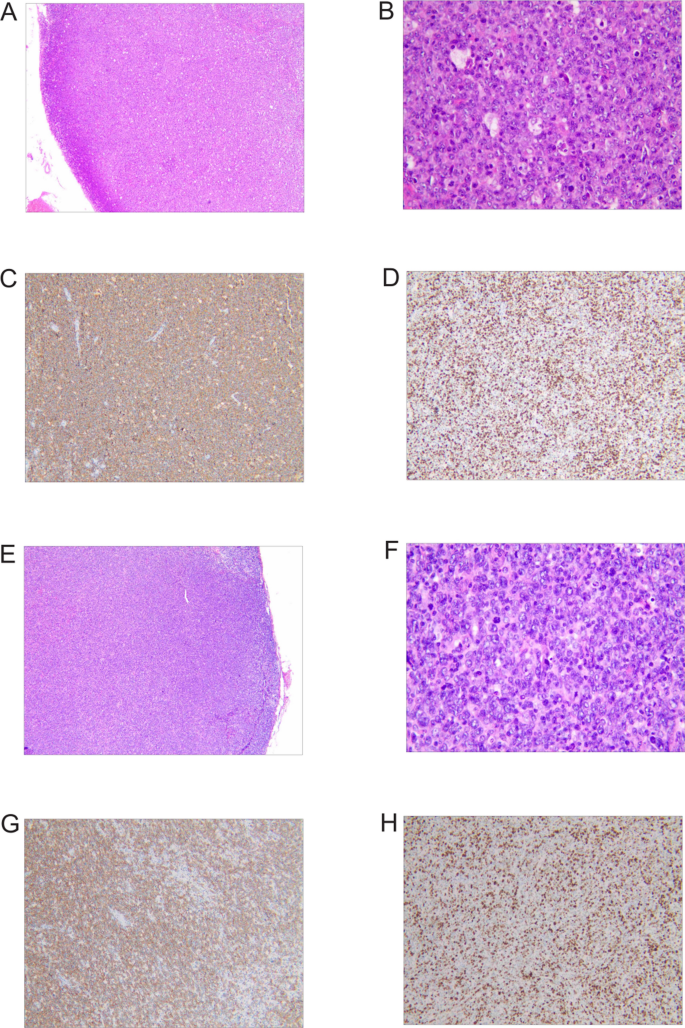
On 11 March 2025, a biopsy of the left cervical lymph node was performed. Pathology indicated diffuse large B cell lymphoma (DLBCL), nongerminal center B cell type (Non-GCB). Immunohistochemical analysis showed the following profile: CD20 (+), PAX-5 (+), Bcl-6 (+), MUM-1 (+, approximately 60% of cells), CD10 (−), Bcl-2 (+, approximately 50% of cells), C-myc (+, approximately 40% of cells), CD3 (−), CD5 (−), CD30 (−), ALK (−), and Ki-67 (+, approximately 80% of cells). In situ hybridization for Epstein–Barr virus (EBER-ISH) was negative. Given the positivity for C-myc (~40%) and Bcl-2 (~50%) in this case, fluorescence in situ hybridization (FISH) was conducted, revealing no BCL6 or BCL2 gene rearrangements and no MYC gene translocation. The patient was definitively diagnosed with diffuse large B cell lymphoma, nongerminal center B cell subtype, double-expressor phenotype, Ann Arbor stage IIIA, and classified as high-risk with an age-adjusted International Prognostic Index (aaIPI) score of 3 (Fig. 1A–D).
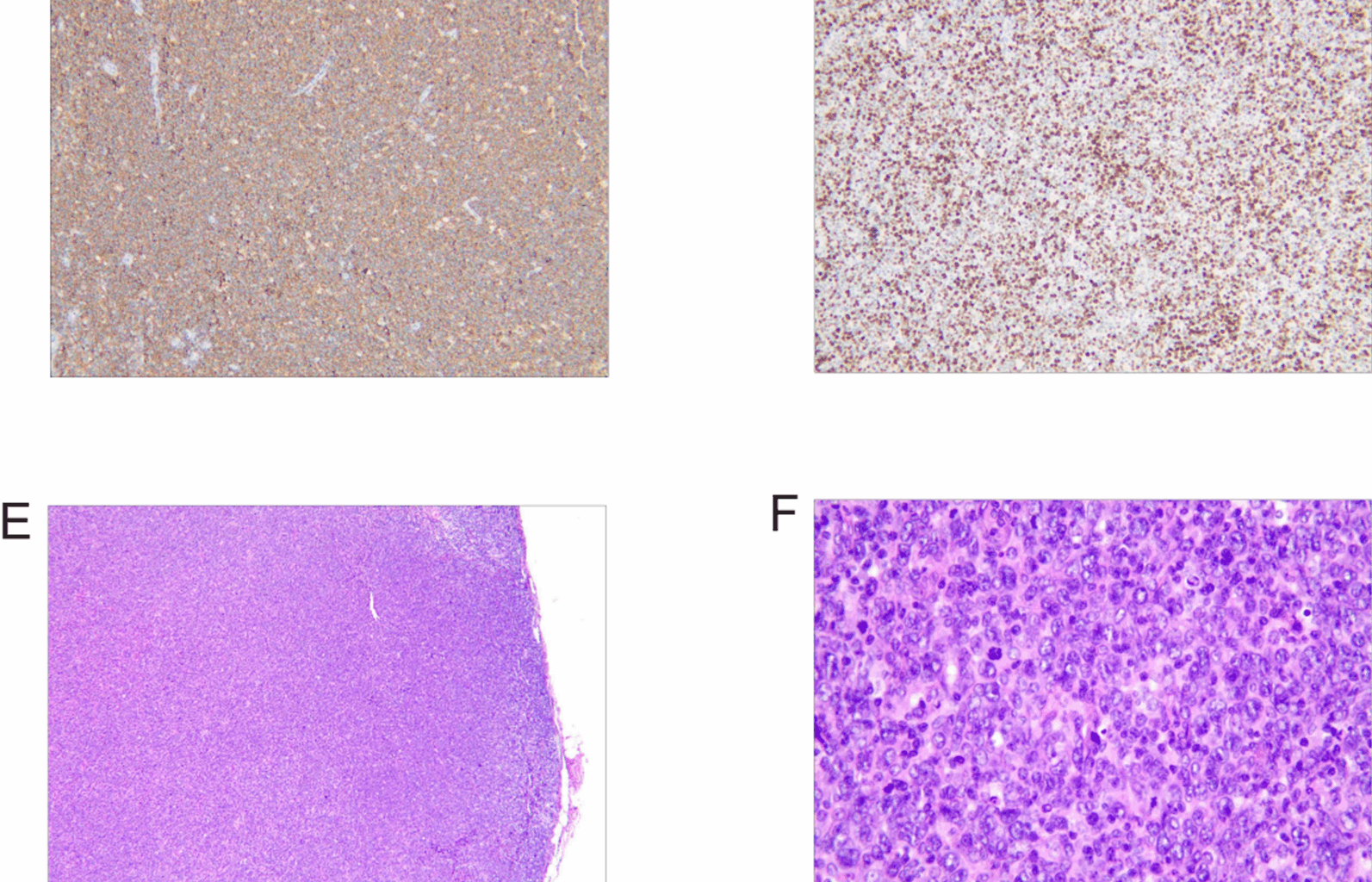
Pathological features of lymph node specimens. A–D Biopsy of the left cervical lymph node from a 29-year-old Han male patient, indicating diffuse large B cell lymphoma (DLBCL), nongerminal center B cell type: A Hematoxylin-eosin staining × 40; B Hematoxylin-eosin staining × 400; C Immunohistochemistry staining showing positivity for CD20; D Immunohistochemistry staining showing positivity for Ki67. E–H Excision of the left inguinal lymph node from a 37-year-old Han female patient, indicating a hematopoietic and lymphoid system tumor: E Hematoxylin-eosin staining × 40; F Hematoxylin-eosin staining × 400; G Immunohistochemistry staining showing positivity for CD20; H Immunohistochemistry staining showing positivity for Ki67
Treatment plan
After admission, the patient was initially treated with prednisone but showed poor response. Blood typing could not be determined, and antiglobulin testing failed. The transfusion department reported cold agglutination at 4 °C in the patient’s blood specimen. Rituximab 100 mg was subsequently administered intravenously once weekly for four doses to eliminate abnormal B cell clones. Following definitive diagnosis of DLBCL, the patient received chemotherapy with the Pola-R-CHP regimen: polatuzumab vedotin 1.8 mg/kg on day 0, rituximab 375 mg/m2 on day 1, cyclophosphamide 750 mg/m2 on day 2, doxorubicin 50 mg/m2 on day 2, and 100 mg of prednisone on days 2–6. Each cycle was administered every 3 weeks for a total of three cycles.
Outcome and follow-up
After the third cycle of chemotherapy, the patient’s fatigue and cyanosis significantly improved. Hemoglobin increased to 111 g/L, reticulocyte percentage decreased from 13.15% to 2.66%, indirect bilirubin declined to 10.8 µmol/L, and lactate dehydrogenase dropped to 410 U/L. Cold agglutinin titer at 4 °C decreased to 1:64. Follow-up contrast-enhanced CT after three cycles showed all lymph nodes with short-axis diameters less than 10 mm, confirming partial remission (PR). The patient is currently under ongoing treatment and regular follow-up.
Case 2: WAIHA combined with DLBCL
We also report the case of a 37-year-old Han female patient who began experiencing dizziness and fatigue without obvious cause more than 5 years ago, with symptoms worsening upon exertion. She was diagnosed with warm autoimmune hemolytic anemia (WAIHA) at a local hospital, and initially responded well to corticosteroid therapy. In August 2023, her symptoms recurred, and she subsequently received prednisone, cyclophosphamide, and rituximab treatment, during which her hemoglobin (Hb) level remained around 80 g/L. On 20 August 2024, owing to worsening dizziness and fatigue, she presented to our hospital. Complete blood count revealed: white blood cell count of 2.51 × 109/L, hemoglobin of 53 g/L, and platelet count of 66 × 109/L. Biochemistry showed total protein of 58.4 g/L, total bilirubin of 73.3 µmol/L, and unconjugated bilirubin of 73.0 µmol/L. She was again diagnosed with hemolytic anemia and received 20 mg of intravenous immunoglobulin (IG) daily for two days, along with folic acid and vitamin B12 supplementation, transfusion of washed red blood cells, and antimicrobial therapy with meropenem plus levofloxacin. On 31 March 2025, she was re-admitted with “dizziness and generalized fatigue for more than 5 years, worsened for 1 week.”
Physical examination
The patient was alert and oriented, with features of severe anemia. Mild scleral icterus was observed. There were no petechiae on the skin or mucosa. No superficial lymphadenopathy was palpable. No tenderness over the sternum. Cardiopulmonary examination was unremarkable. The abdomen was soft; the spleen was palpable 2 cm below the left costal margin without tenderness; the liver was not palpable below the costal margin. No edema was noted in either lower limb.
Laboratory examinations
Complete blood count showed: white blood cell count of 1.00 × 109/L, red blood cell count of 1.92 × 1012/L, hemoglobin of 55 g/L, platelet count of 88 × 109/L, reticulocyte percentage of 13.34%, and absolute reticulocyte count of 0.2228 × 1012/L. Biochemistry: total bilirubin of 85.5 µmol/L, direct bilirubin of 8.9 µmol/L, indirect bilirubin of 76.6 µmol/L, and lactate dehydrogenase (LDH) of 505 U/L. Coagulation panel: prothrombin time was 12.3 seconds, activated partial thromboplastin time was 30.8 seconds, fibrinogen was 4.55 g/L, D-dimer was 0.85 µg/mL, and fibrin degradation products were 4.9 µg/mL. Pretransfusion immunological testing showed blood type O RhD positive, with a positive irregular antibody screen. The direct antiglobulin test was positive (IgG positive), and the indirect antiglobulin test was also positive. Immunoglobulins and complement levels, serum immunofixation electrophoresis, serum protein electrophoresis, serum free light chain assay, paroxysmal nocturnal hemoglobinuria (PNH) clone detection, extended immunologic panel, routine urinalysis, and stool routine with occult blood test showed no significant abnormalities. Bone marrow cytology revealed active nucleated cell proliferation with a granulocyte-to-erythroid ratio of 1.48:1. Granulocytic lineage showed morphologically normal cells at various stages and presence of eosinophils. The erythroid lineage displayed a slightly elevated proportion of late erythroblasts with overall normal morphology. Mature erythrocytes were anisocytotic. Lymphocyte ratio was within normal range with unremarkable morphology. A total of 219 megakaryocytes were observed across the smear, including 25 classified cells: 3 promegakaryocytes, 8 platelet-producing megakaryocytes, 13 granular megakaryocytes, and 1 naked nucleus. Platelets were seen scattered and in clusters, with a relatively abundant count. Bone marrow immunophenotyping showed no evidence of acute leukemia, high-risk myelodysplastic syndromes (MDS), lymphoma, or plasma cell myeloma-associated immunophenotypic abnormalities. Bone marrow biopsy revealed no increase in B lymphocytes. The lab results were presented in Table 2.
Table 2 Lab results of case 2Imaging studies
Hepatobiliary, pancreatic, and splenic ultrasound revealed splenomegaly (spleen thickness 77 mm, 24 mm below the costal margin) with multiple nodules. Noncontrast and contrast-enhanced CT of the entire abdomen showed multiple splenic nodules, multiple enlarged retroperitoneal lymph nodes (short-axis diameter 15–20 mm), a hypodense nodule in the left hepatic lobe (follow-up recommended), and a right renal cyst. Superficial lymph node ultrasound demonstrated abnormal enlargement of bilateral cervical, submandibular, supraclavicular, axillary, and inguinal lymph nodes (maximum short-axis diameter 12 mm), with partial loss of hilar structure.
Pathology and immunohistochemistry
On 11 March 2025, a biopsy of the left cervical lymph node was performed. Pathology indicated diffuse large B cell lymphoma (DLBCL), nongerminal center B cell type (Non-GCB). Immunohistochemical analysis showed the following profile: CD20 (+), PAX-5 (+), Bcl-6 (+), MUM-1 (+, approximately 60% of cells), CD10 (−), Bcl-2 (+, approximately 50% of cells), C-myc (+, approximately 40% of cells), CD3 (−), CD5 (−), CD30 (−), ALK (−), and Ki-67 (+, approximately 80% of cells). In situ hybridization for Epstein–Barr virus (EBER-ISH) was negative. Given the positivity for C-myc (~40%) and Bcl-2 (~50%) in this case, fluorescence in situ hybridization (FISH) was conducted, revealing no BCL6 or BCL2 gene rearrangements and no MYC gene translocation. The patient was definitively diagnosed with diffuse large B cell lymphoma, nongerminal center B cell subtype, double-expressor phenotype, Ann Arbor stage IIIA, and classified as high-risk with an age-adjusted International Prognostic Index (aaIPI) score of 3 (Fig. 1A–D).
Treatment plan
After admission, the patient received intravenous human immunoglobulin (20 g/day for 7 days) to ameliorate hemolytic anemia, granulocyte colony-stimulating factor to elevate white blood cell counts, cefoperazone-sulbactam for infection prophylaxis, and washed red blood cell transfusions to correct anemia. Upon DLBCL diagnosis, Pola-R-CHP chemotherapy was initiated: polatuzumab vedotin 1.8 mg/kg on day 0, rituximab 375 mg/m2 on day 1, cyclophosphamide 750 mg/m2 on day 2, doxorubicin 50 mg/m2 on day 2, and prednisone 100 mg on days 2–6, administered every 3 weeks for two cycles.
Outcome and follow-up
After the second chemotherapy cycle, the patient exhibited significant improvement in fatigue and jaundice. Hemoglobin levels gradually increased to 75 g/L, reticulocyte percentage decreased to 6.72%, indirect bilirubin dropped to 12.6 μmol/L, and lactate dehydrogenase declined to 257 U/L. Post-two-cycle CT re-examination showed a 50% reduction in splenic nodules, with enlarged lymph nodes measuring