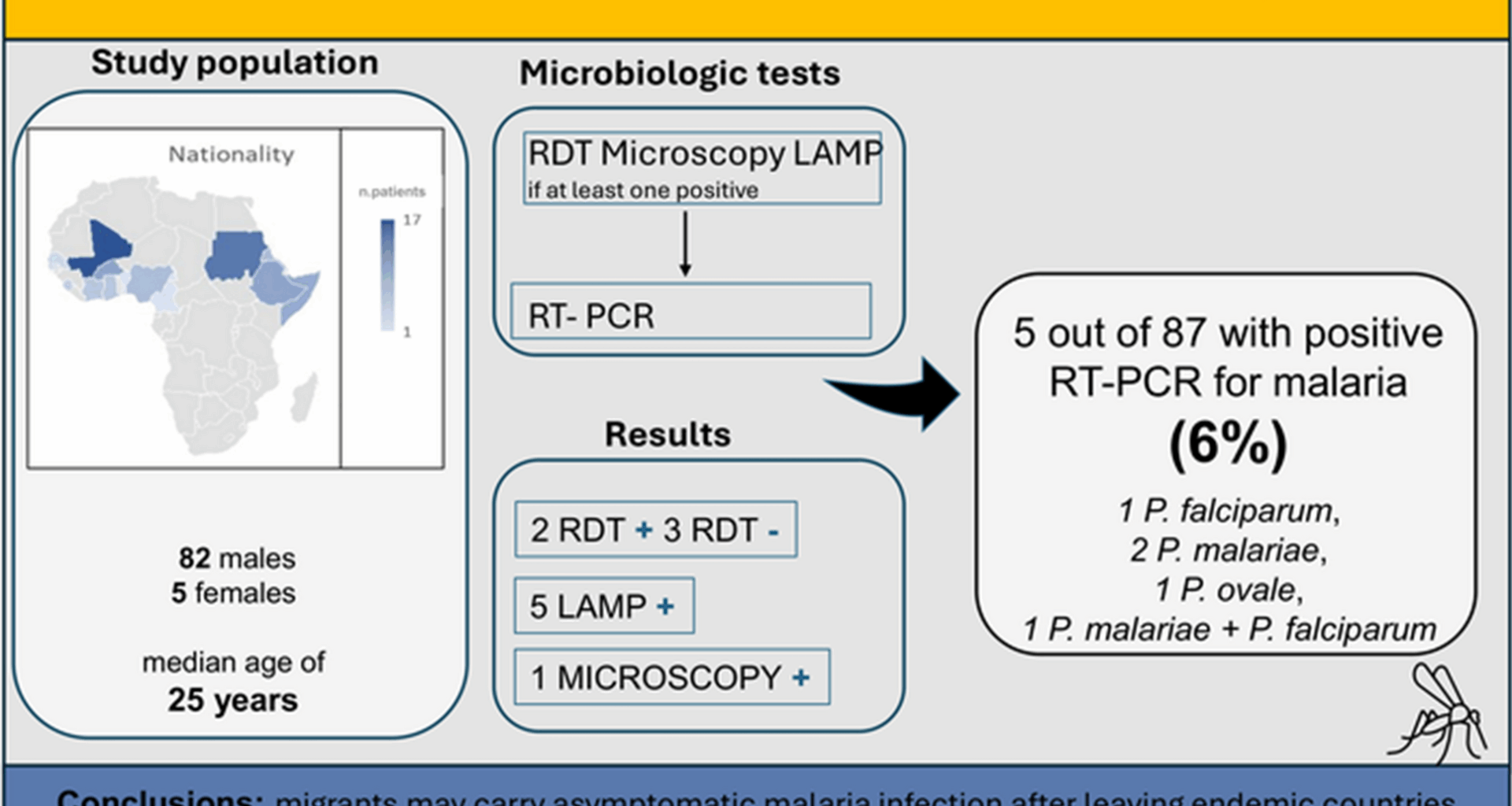
Study design and study population
Since February 2024, an adult outpatient clinic at the Lazzaro Spallanzani National Institute for Infectious Diseases has been dedicated to serve migrants, especially the mobile population residing in urban and suburban areas of Rome. The clinic is conducting an infectious disease screening program (February 2024–January 2025), including tests for Schistosoma, hepatitis B and C virus (HBV and HCV), and human immunodeficiency virus (HIV), and interferon gamma release assay (IGRA) test. Malaria screening with rapid diagnostic test (RDT), loop-mediated isothermal amplification (LAMP), thick blood smear, and real-time polymerase chain reaction (RT-PCR) assay was offered to asymptomatic patients from endemic regions in SSA or those with migration routes through these areas, regardless of their duration of residence in Italy. Participants gave informed consent, with translators’ assistance when necessary. Exclusion criteria included origin from non-endemic regions, age under 16, and fever at the time of testing.
Patients with positive results were referred to the in-patient clinic for evaluation and antimalarial drug therapy. During the clinical visit we collected data about the origin country, date of departure, migration route, duration of migration, method of travel to reach Italy, time spent in detention centres if the case, arrival date in Italy, co-morbidities and medications, previous malaria infections, specific treatments, as well as any persistent symptoms at the time of evaluation.
Detection of malaria parasites
Malaria screening was conducted using a combination of antigen-based RDTs, microscopy, and LAMP, while RT-PCR was subsequently performed for confirmation.
RDTs (CareUs Malaria Rapydtest® Combo Pf/PAN, Wells Bio, Inc., Seoul, Republic of Korea, distributed in Europe by Apacor Ltd. Wokingham, United Kingdom), were performed on sampling day to detect P. falciparum, P. vivax, P. malariae, or P. ovale. If positive for non-P. falciparum species, an additional rapid test (Screen Test Malaria P.F./P.V. rapid test; Screen Italia, Perugia, Italy) was conducted to differentiate P. vivax.
For samples that tested negative by RDT, DNA extraction was performed on the blood cell fraction and analyzed using the Alethia® Malaria assay (Meridian Diagnostics, Cincinnati, OH, USA), a LAMP method for rapid detection of Plasmodium spp. Samples that were positive by either RDT or LAMP underwent further testing with a real-time PCR assay (Malaria Differentiation REAL TIME PCR Detection Kit—Certest Biotec®, Zaragoza, Spain) to identify specific Plasmodium species, including P. falciparum, P. vivax, P. ovale, P. malariae, and P. knowlesi.
Data analysis
Descriptive statistics were used to summarize epidemiological, clinical, and laboratory data. Continuous variables with normal distribution were expressed as means ± standard deviation (SD), while non-normally distributed variables were reported as medians with interquartile ranges (IQR). Categorical variables were presented as counts and percentages. Statistical analyses were performed using JASP (version 0.18.3; University of Amsterdam, Amsterdam, The Netherlands).