As a distinct subtype of adenocarcinoma, PMA’s CT manifestations are closely related to the pathological characteristics of tumor mucin secretion. Pneumonia-like patchy opacities or large areas of consolidation are commonly observed, with often poorly defined boundaries, showing infiltrative growth and forming halo signs or reverse halo signs [7]. The mucoid bronchial sign in the consolidation area presents typical tree-in-bud changes, with bronchial dilation accompanied by intraluminal mucus plugs, and bronchial wall stiffness and truncation. Post-contrast images demonstrate no enhancement. This triad—comprising bronchial dilation, mucus plugging, and absence of wall enhancement—exhibits high specificity [8]. Some cases may present as single or multiple nodules or masses. Single lesions are rounded with smooth edges, and internal nodular mucin retention can form bubble signs. Multiple lesions present as randomly distributed snowflake signs in both lungs, some with punctate calcification caused by calcium salt deposition in mucin [9].
This case’s CT manifestations differ significantly from typical PMA, showing bilateral diffuse patchy opacities and consolidation with multiple cavities and nodules, similar to common bacterial pneumonia, organizing pneumonia, pulmonary fungal infection (such as Aspergillus), or pulmonary lymphoma, which can readily lead to clinical misdiagnosis [10]. Cavities are common in various lung diseases, including pneumonia, lung abscess, tuberculosis, lung squamous cell carcinoma, and pulmonary metastases, but are relatively rare in pulmonary mucinous adenocarcinoma. Research indicates that cavitary lesions in PMA patients occur in only 3–10% of cases. The presence of cavitation in PMA carries significant prognostic implications that warrant special attention. As demonstrated in previous studies, patients with cavitary PMA have markedly worse outcomes compared to their non-cavitary counterparts, with 5-year survival rates dropping to 22% versus 48% for non-cavitary patients [11]. This survival disparity may be attributed to several factors: First, cavitation often signifies a more aggressive tumor biology characterized by increased mucin production and tissue destruction; it is frequently associated with more advanced disease stages at diagnosis; and the cavitary pattern may represent a more invasive phenotype with increased metastatic potential. Furthermore, the atypical imaging appearance of cavitary PMA, as exemplified by our case, often leads to diagnostic delays during which the disease progresses, potentially contributing to the poorer prognosis. These findings underscore the critical importance of maintaining high clinical suspicion for malignancy in patients presenting with cavitary lung lesions, particularly when infectious etiologies have been adequately excluded.
A literature search was conducted using PubMed and other databases with the keywords “Pulmonary mucinous adenocarcinoma,” “CT features,” and “cavitation.” Relevant literature published within the last 10 years was selected to summarize characteristics of CT manifestations of PMA and compare them with this case (Table 1).
Table 1 Comparative analysis of typical CT manifestations of pulmonary mucinous adenocarcinoma and this case
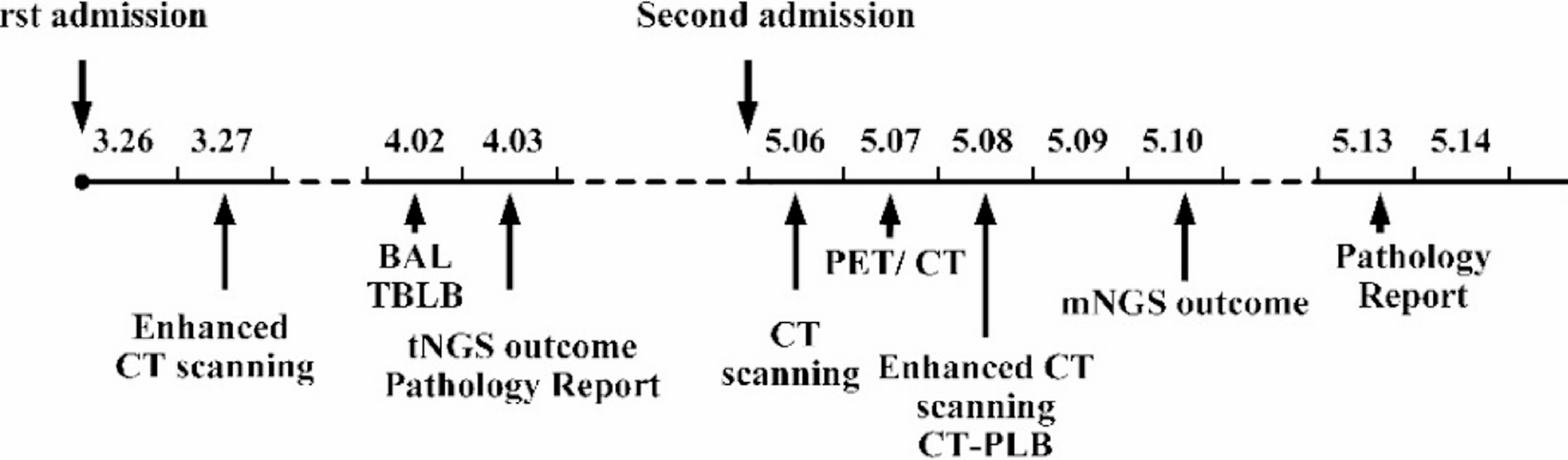
In this case, due to atypical CT manifestations and bronchoscopic lung biopsy pathology showing only mild atypical mucinous cell proliferation in the alveolar wall with low cell count, no clear evidence for PMA diagnosis was found. Finally, PMA was diagnosed through CT-guided lung puncture biopsy. This case offers an important clinical insight: in patients diagnosed with “pneumonia” who exhibit a poor response to anti-infective therapy or experience recurrent symptoms, the possibility of malignancy should be considered, especially when CT shows mixed cavities and nodules, requiring dynamic observation of imaging changes.
Regarding the role of PET/CT in this diagnostic workup, although it can sensitively detect metabolically active lesions, its utility in distinguishing between malignant and infectious processes remains limited, particularly before obtaining definitive tissue diagnosis [12]. In this case, the high SUV values (SUVmax 10.0) demonstrated significant metabolic activity but could not reliably distinguish between active infection and malignancy, as both conditions can exhibit similar FDG uptake patterns. The decision to perform PET/CT was based on the need to assess disease extent and metabolic activity in the context of progressive symptoms and elevated tumor markers; however, the ultimate diagnostic determination relied on histopathological confirmation rather than metabolic imaging findings alone.
As with other lung cancers, histopathological examination remains the gold standard for diagnosing PMA. In clinical practice, bronchoscopic lung biopsy is a commonly employed method for obtaining pathological samples; however, due to constraints such as limited operating space, diagnostic yield may be suboptimal owing to inadequate sampling. CT-guided lung puncture biopsy can improve diagnostic yield, especially for peripheral lesions [13].
Treatment of pulmonary mucinous adenocarcinoma should follow individualized comprehensive treatment principles based on tumor staging, molecular characteristics, and pathological subtypes [14]. Early-stage disease is managed with surgical radical treatment; locally advanced inoperable disease is treated with concurrent chemoradiotherapy combined with immunotherapy consolidation; and advanced disease management emphasizes molecular typing-driven precision strategies [15]. This patient was diagnosed with advanced-stage PMA. In the absence of further testing for gene mutations or PD-L1 expression, a regimen of cisplatin, pemetrexed, and anlotinib was administered for anti-angiogenic therapy [16].
The clinical implications of this case extend beyond its rarity and emphasize fundamental principles of diagnostic vigilance. When patients present with pneumonia-like lesions that demonstrate poor response to appropriate antibiotic therapy, especially in the setting of progressive symptoms, enlarging lesions, or elevated tumor markers, clinicians should promptly consider malignancy and pursue tissue diagnosis. The threshold for pursuing invasive diagnostic procedures should be lower in such scenarios, as early histopathological confirmation can prevent prolonged inappropriate treatment and ensure timely initiation of appropriate oncological management. This case reinforces that atypical imaging presentations should not deter clinicians from pursuing definitive tissue diagnosis when clinical suspicion for malignancy exists.