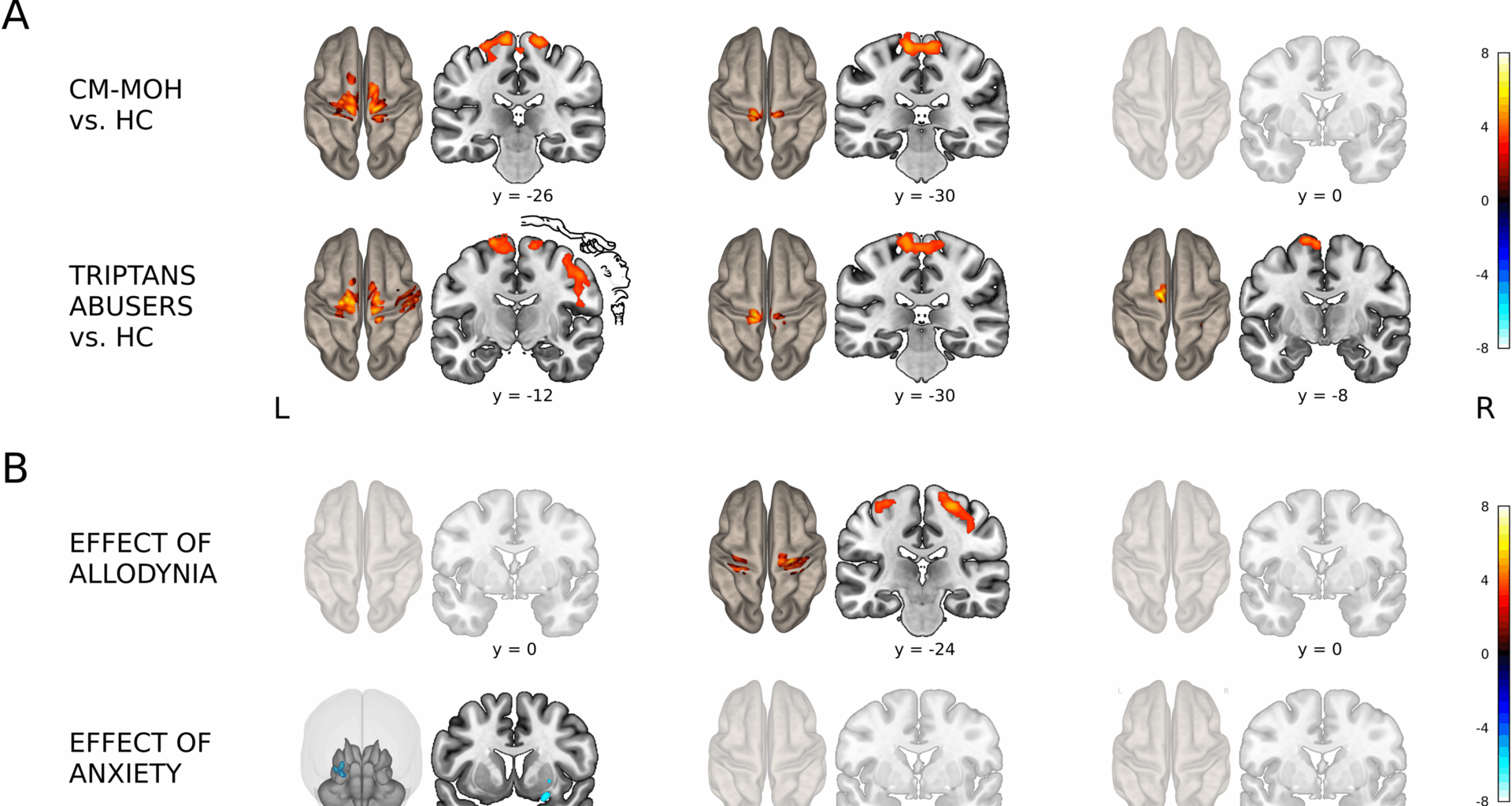
Our research explored the resting-state FC of the triple network model (DMN, SN, and CEN) and their interactions with brain regions in a large sample of CM-MOH patients (N = 91) compared to healthy controls. Taken together, our findings indicate that CM-MOH patients exhibit alterations across three complementary levels of connectivity: interactions of the triple network model with extra-network regions, inter-network connectivity within the triple network model, and intra-network efficiency of specific nodes. In CM-MOH patients, the DMN, which supports self-referential thinking, and the SN, crucial for detecting relevant sensory and emotional stimuli, both exhibited increased FC with primary sensorimotor areas. These alterations were particularly pronounced in triptan abusers. Moreover, several nodes of the SN showed reduced global and local efficiency, suggesting a loss of integration and specialization within the triple network model. The CEN, responsible for high-cognitive tasks, also showed connectivity changes with motor regions in triptan abusers, while NSAID abusers and polyabusers exhibited a similar but less pronounced subthreshold pattern. With respect to clinical variables, greater allodynia scores were associated with increased SN-sensorimotor connectivity and with decreased DMN intra-network connectivity in parietal nodes, while higher anxiety scores correlated with decreased DMN-amygdala-putamen connectivity. Increased medication intake and migraine chronification were linked to enhanced FC of both DMN and SN with multisensory regions, including temporal and occipital areas. Notably, polyabusers, showed inter-network connectivity disruptions between the SN and CEN, in frontal and parietal nodes and concurrently enhanced coupling of both networks with temporo-occipital regions as an effect of medication intake.
Intra- and inter-network alterations within the triple network model
Previous studies have reported both intra-network (DMN, SN, CEN) [23,24,25, 27] and inter-network [26, 27] FC alterations in chronic migraine, with more pronounced SN and CEN intra-network disconnection in CM-MOH [23, 25]. In line with these findings, our results in CM-MOH show intra-network disruption in the SN and DMN and extend current evidence by highlighting possible inter-network SN–CEN alterations that seem to be specific to polyabusers. Notably, these patients are at higher risk of developing CM-MOH compared with single-drug users (NSAIDs or triptans) [15] and may be more prone to an abuse-like profile. The altered SN–CEN inter-network connectivity resembles patterns previously reported in substance abuse and addiction studies within the triple network model [39, 40]. This finding suggests a possible role of the CEN in drug abuse behaviour, whose impaired executive control functions may reduce top-down regulation of the SN, thereby facilitating maladaptive salience attribution to medication-related cues.
Interplay among networks of the triple model and sensorimotor regions
Our results revealed aberrant FC of DMN and SN with somatosensory regions in CM-MOH patients compared to healthy controls, consistent with findings in other chronic pain conditions, such as fibromyalgia and low back pain, which show increased inter-network connectivity among DMN, SN, and the sensorimotor network [34, 51,52,53,54,55]. These alterations may impair the discrimination, perception, and processing of painful stimuli [22]. Notably, enhanced FC between the DMN, related to internal awareness, and somatosensory regions may contribute to the embodiment of chronic pain, integrating it into self-perception [35, 54]. This hypothesis is supported by previous evidence showing that FC between DMN and primary somatosensory cortex intensifies in relation to pain persistence [33]. On the other side, increased FC between the SN, encoding sensory, autonomic, and emotional information, and the somatosensory cortex may amplify the unpleasant experience during emotional pain processing in CM-MOH patients [33]. Importantly, SN-sensorimotor connectivity is positively associated with allodynia symptoms, a known risk factor for migraine chronification [14]. These findings are consistent with previous studies showing increased allodynia severity linked to enhanced resting-state FC of SN in CM patients [24] as well as increased FC of the secondary somatosensory cortices in episodic migraine patients [56].
Functional characterization by abuse profile
Our results suggest that the altered FC pattern observed across the entire sample is primarily driven by triptan abusers. Compared to healthy participants, these patients exhibited a broader pattern of increased DMN and SN connectivity with sensorimotor regions along with altered CEN-Supplementary Motor Area (SMA) connectivity. Notably, these alterations were also present in NSAIDs abusers and polyabusers, albeit with a lower statistical threshold, suggesting a common pattern along abuse profiles, that is more pronounced in triptans abusers.
These results could be related with chronic pain that may enhance DMN connectivity in the neural regions representing the pain-affected face, aligning with a somatotopically-dependent pattern of pain representation. This is consistent with studies showing region-specific DMN and SN connectivity changes in chronic pain patients based on pain localization [55, 57], although in our study such alterations were mainly observed for the DMN and only appeared in the unthresholded maps for the SN.
In addition, alterations within the CEN have been previously reported in both chronic [24] and episodic migraine patients [22, 58, 59]. This heightened CEN-SMA coupling may potentially reflect altered goal-directed behaviour and motor defensive strategies, and potentially impair higher-order cognitive functions [23].
Among abuse profiles, triptan abusers exhibited the most marked FC alterations that might likely reflect the distinct mechanisms of action and prescription indications of drugs of abuse [17, 18]. Triptans specifically target migraine pathways, are generally prescribed as second-line treatments for severe attacks, and carry a higher and faster risk of CM-MOH development [60,61,62], factors that together may contribute to the widespread FC alterations observed across all networks of the triple model. Conversely, NSAIDs exert a more generalized, non-specific effect, are recommended as first-line treatment for acute migraine [60,61,62], and these features may account for the subtler FC abnormalities.
Impact of migraine chronification and drug intake on functional connectivity
Several demographic and clinical factors increase the risk of migraine chronification [2, 14, 24] and drug overuse [63]. We found that CM duration was positively associated with increased SN and DMN connectivity with the occipital cortex, while drug intake correlated with increased DMN connectivity with temporal regions. These patterns suggest that repeated drug intake and longer CM duration may enhance sensory processing and plasticity in multisensory regions, promoting drug-related cue representation, similar to other substance abuse disorders [64,65,66]. Moreover, CM duration often includes remission phases, highlighting its cyclical nature and relapse tendency, which may reinforce altered network interactions and drug-seeking behaviors. Indeed, previous studies have implicated occipital pole FC and excitability in distinguishing chronic migraines from episodic migraine [67], as well as in remission of CM to episodic ones [68]. In line with this, visual cortex dysfunction is considered a key feature of migraine, both during and between attacks [69]. Our observations might suggest that persistent high-frequency migraine attacks, along with higher drug intake, may disrupt DMN and SN interactions with visual and temporal regions, potentially worsening migraine chronification. On the other hand, repeated drug use may sensitize mesocorticolimbic systems [62, 70], which closely overlap with the SN [71]. In polyabusers, increased drug intake is associated with more widespread FC alterations, particularly between the SN and CEN and bilateral temporo-insular regions. Over time, this sensitization could increase the salience of medication-related cues, triggering physiological arousal and attentional biases that drive drug-seeking behaviour [62, 65]. Consistent with these functional modulations, the inter-network SN–CEN disconnection observed in polyabusers may reflect reduced executive control over salience processing, a mechanism that implicated in substance abuse disorders and potentially contributing to compulsive medication-seeking in MOH.
Association with psychiatric symptoms
State anxiety was negatively associated with DMN connectivity to a subcortical cluster encompassing the right amygdala, known for its role in emotional regulation, and part of the right putamen. In patients with sustained anxiety, the amygdala negatively couples with DMN FC [33, 72, 73]. We can speculate that when pain becomes embodied, leading to abnormal DMN connectivity with sensory processing regions, it may trigger anxiety, or conversely, anxiety may exacerbate pain perception and disrupt DMN connectivity. This maladaptive modulation can impair emotional regulation, further worsening patients’ well-being and mental health.
Strengths and limitations
Our study has some limitations. First, the absence of a CM patient group prevents us from determining whether the observed characteristics are specific to MOH. However, it should be noted that previous studies observed similar patterns of intra-network FC alterations in CM and CM-MOH patients, albeit with more pronounced dysconnectivity in SN and CEN in CM-MOH [23,24,25]. Additionally, as a tertiary centre, our population predominantly consists of MOH cases, limiting generalizability. Second, the cross-sectional design and the chronic nature of the disease preclude causal inference on pain-related connectivity changes. Third, we did not collect data on the frequency of specific headache types (e.g., migraine vs. tension-type), which may differ across drug abuse profiles. A further limitation is the absence of specific questionnaires assessing substance abuse, which would have provided a more direct characterization of addiction-related behaviours in MOH patients. Future studies should validate these findings in episodic migraine and CM patients to clarify specific features related to chronicity and MOH.
Nonetheless, our study has notable strengths. The large sample size enhances statistical power and robustness of our conclusions, while detailed pharmacological characterization provides insights into drug effects. Similar depression and anxiety scores across abuse profiles ensure comparability of psychiatric variables. Importantly, our investigation encompasses not only intra-network connectivity but also inter-network and whole-brain connectivity analyses, providing a comprehensive perspective.