Researchers have traced where and when the HIV protective mutation arose but also how it gave northern European ancestors a competitive advantage against ancient diseases
A genetic mutation in a multi-millennia-old human – who lived and died around the Black Sea region in the Western Eurasian Steppe – has influenced the design of modern HIV medicines.
Research from the University of Copenhagen has found that up to a quarter of the population of Denmark have a genetic mutation that makes them resistant – or even immune – to HIV. This knowledge has been deployed in the creation of modern drugs against the virus.
Where and when this mutation arose was unknown until now. Or, indeed, why it occurred at all. But this genetic mystery has now been solved with some advanced DNA research and technology.
“HIV is a relatively new disease – [emerging] less than 100 years ago – so it’s almost coincidental and very fascinating that a genetic variation that arose thousands of years ago [can] also protect against a modern virus like HIV,” said Professor Simon Rasmussen from the Novo Nordisk Foundation Centre for Basic Metabolic Research (CBMR) at the University of Copenhagen, and corresponding author for the study that has mapped the mutation.
“[We found that] the variant arose in one individual who lived in an area near the Black Sea between 6,700 and 9,000 years ago,” he added.
But, in order to determine the historical and geographical origins of the mutation, the team mapped its progress by analysing the DNA of around 2,000 living people worldwide. And then coupled this dataset with an AI-based methodology to identify the mutation in the DNA that was recovered from ancient bones.
More than 900 skeletons were examined that dated back as far as the Stone Age – specifically the final dataset encompassed consecutive historical eras, ranging from the early Mesolithic (~10,000 to ~4,000 BCE) through the Neolithic period to the Bronze Age and extending into the Viking Age (~800 CE to ~1,100 CE).
“[From] this large dataset, we determined where and when the mutation arose. For a period, the mutation is completely absent, but then it suddenly appears and spreads incredibly quickly.
“When [overlayed] with our knowledge of human migration at the time, we can also pinpoint the region where the mutation originated,” explained Dr. Kirstine Ravn, senior researcher at CBMR, and first author of the paper, which led to the identification of the Black Sea individual, all of whose all descendants are carriers of the mutation.
The researchers believe the once the mutation arose it spread rapidly because it afforded the ancestors of the Danes a survival advantage. At this time humans were migrating in a pattern from the Western Eurasian Steppe, east-to-west, and then north into the Danish peninsula.
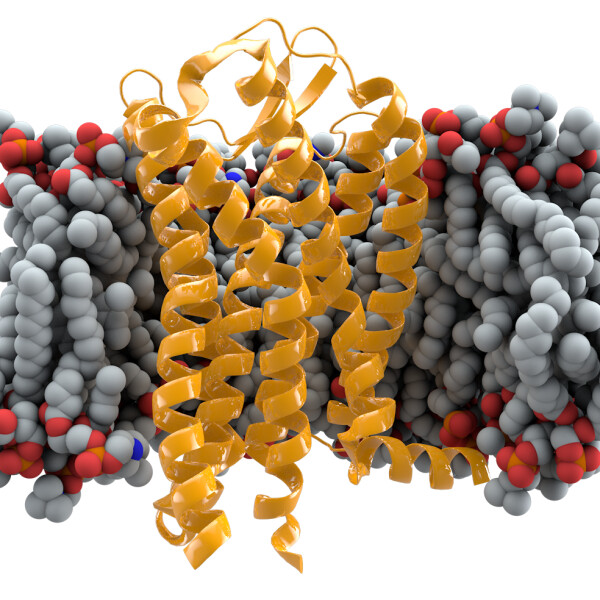
The genetic change in question is the deletion of CCR5delta32. The CCR5delta32 allele frequency (AF) is up to 0.16 in northern European populations but found half as frequently at less than 0.08 in southern Europe, and in the rest of the world is only found in populations with a northern European ancestry.
CCR5 – a cysteine-cysteine chemokine receptor type 5 – is a protein found on the surface of white cells that is involved in the immune system by promoting cellular migration by chemotaxis. It acts as receptors for chemokines – a type of cytokine – and the deletion can down moderate the immune response known as a cytokine storm. Primary HIV infection sometimes triggers a sudden and severe inflammatory process consistent with cytokine storm syndrome.
“People with this mutation were better at surviving. This is likely because [the mutation] dampened the immune system during a time when humans were exposed to [many] new pathogens,” explained Dr. Leonardo Cobuccio, post-doctoral researcher at CBMR, and co-first author.
“[But] what’s fascinating is that the variation disrupts an immune gene. It sounds negative, but it was likely beneficial. An overly aggressive immune system can be deadly – think of allergic reactions or severe cases of viral infections like COVID-19, where the immune system often caused the damage that killed patients.
“As humans transitioned from hunter-gatherers to living closely together in agricultural societies, the pressure from infectious diseases increased, and [so] a more balanced immune system may have been advantageous,” he added.
For further reading please visit: 10.1016/j.cell.2025.04.015