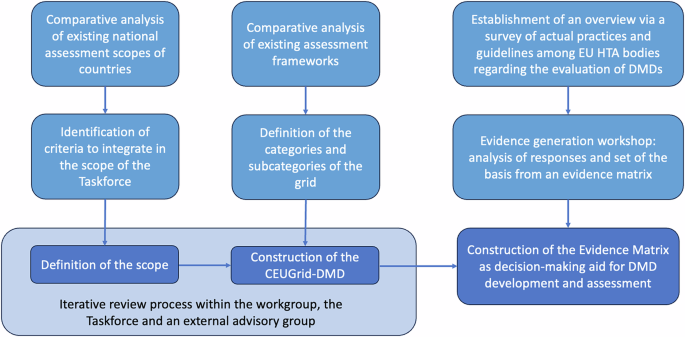
Definition of the scope of the grid
The selected criteria included in the scope of the grid were the following: (a) CE marking devices, (b) devices whose main function is based on a digital technology, (c) devices whose functionalities can serve a variety of purposes: prevent, diagnose, monitor, treat (therapeutics), clinical decision management (i.e., health care pathway), treatment management decision-making, and (d) device whose users can be one of the following: HealthCare Professionals (HCP), patients, caregivers, health system users. Exclusion criteria were the following: devices not intended to support medical purposes, software qualified as an accessory for a hardware and administrative software. Including only CE-marked devices permitted to clearly differentiate wellness applications from other applications having a medical purpose, an approach coherent with assessment procedures where clinical evidence is required solely for devices with a medical purpose.
These criteria also enable to propose a specific terminology for the technologies in scope and defined as DMDs. These can thus be considered as a sub-category of Digital Health Technologies (encompassing technologies that are not medical devices such as wellness applications) which have a medical purpose and are therefore medical devices. Applying all inclusion and exclusion criteria has led to the adoption by consensus of the following definition of the scope: Digital Medical Devices are health technologies falling into the definition of Medical Devices as outlined in the Regulation (EU) (2017/745)7 and whose main function is based on digital technologies intended to support one or more of the following medical purposes:
-
Prevention, diagnosis, monitoring, treatment or alleviation of disease.
-
Diagnosis, monitoring, treatment, alleviation of, or compensation for, an injury or disability.
These devices could include software, hardware, static and self-learning algorithms (e.g. those based on artificial intelligence). DMDs can be used by people or the wider health and social care system. They may include smartphone applications, standalone software, online tools for treating or diagnosing conditions, preventing ill health, or for improving system efficiencies as well as programs that can be used to analyse data from medical devices such as scanners, sensors or monitors. They do not include devices that are not intended to support medical purposes (e.g., wellness applications), software qualified as an accessory for a hardware and administrative software.
This approach ensures an EU-wide application of the grid as it remains in line with the existing national classification schemes and could be applicable regardless the maturity stage of a country in regulating DMDs. Furthermore, coherence with the Medical Device Regulation (EU) 2017/7477 is considered essential.
The Common European Classification Grid for Digital Medical Devices (CEUGrid-DMD)
The CEUGrid-DMD presents the following classification items: DMD category, its function (without distinction of the algorithm nature), the intended beneficiary (patient and/or HCP), context of use, either stand-alone setting or as part of a recognised integrated care pathway (Table 1). Categories for DMDs are established according to their main intended use: “A-Inform”, “B-Diagnose”, “C-Manage”, “D-Monitor” and “E-Treat”. Each category corresponds to one or more sub-categories based on the DMD functionality, the intended beneficiary and the context of use. As such, the category “A-Inform” corresponds to a DMD having prevention and health education as its main function. The Category “B-Diagnose” targets DMDs serving as diagnostic aid by providing information to HCP to take an immediate or near-term action to diagnose, screen or detect a disease or condition. This category does not include DMDs whose objective is to provide to patients a complete diagnosis, as we consider that a DMD never gives a diagnosis directly to a patient. The category C-Manage comprises two distinct DMD functionalities. The first aims to provide support to the organisation of care and enhance efficiency. Examples are triage systems or teleconsultations. The second includes DMD supporting self-management systems which build on personalized information, such as guidance using behaviour change techniques or assistance to mitigate disability and disease symptoms and to promote good health and healthy lifestyles. In these cases, no input from HCPs is required and the information provided by the DMD will not trigger an immediate or near-term action by the HCP. In the category “D-Monitor”, two functional sub-categories are identified. The first includes DMDs enabling self-monitoring of a disability or a disease. Examples are DMDs helping individuals with a diagnosed and treated condition to manage their treatment autonomously, essentially in the context of chronic, somatic or psychiatric illnesses. Other DMDs falling into these sub-categories are those managing a non-pathological health state or DMDs that can be used for rehabilitation by the patient itself. If alerts are provided by these DMDs, they would only target the patients themselves. This is in contrast to the second sub-category of remote monitoring assisting HCP in the management of care and the monitoring of the treatment. Finally, in the category “E-Treatment”, three functional sub-categories distinguish DMDs according to whether they are “support self-treatment”, “treatment aids” or “therapeutic decision-making”. While the first sub-category enables patients to improve the management of their condition, the two others support the HCP. A lot of DMDs have a main intended use, and therefore a main category and functionality, but also one or more other functionalities. Moreover, regarding the type of algorithm embedded, it is a useful information to understand what is being assessed. However, no distinction has been made in the CEUGrid-DMD as it has no consequences on how to assess the clinical or organisational interest of a DMD.
Table 1 Common Classification European Grid for Digital Medical Devices (CEUGrid-DMD)State of the art: responses to the survey by Health Technology Assessment (HTA) bodies and evidence generation workshop
In July 2023, a survey was sent, with the help of the remaining European network for HTA “EUnetHTA”, to 32 national representatives of HTA bodies (18 countries). From July 2023 to April 2025, only Belgium, Germany and France have made DMDs reimbursable. Other countries such as Italy, Finland or Austria are in the process of developing their HTA regulations. Ten HTA bodies in nine European countries answered the survey (Table 2). Some HTA bodies have declined to answer to the survey as their DMD evaluation process was not yet defined. HTA bodies that could respond provided concrete information on the context of assessments (the scope of the assessment, the existence of a specific framework for the assessment of DMDs, their assessment domains) and on the evidence requirements. Most countries that responded to the survey applied traditional Medical Devices HTA frameworks. Only few countries had a specific framework for DMDs in place i.e., Belgium8, Germany9 and France10,11. The latter, however, did not target all DMDs, but regarded only one or two types (e.g., telemonitoring solutions and therapeutic applications). The scope of these specific frameworks was therefore limited. The answers also showed that the assessment domains were not similar between European countries. The only domain evaluated in all countries was the clinical domain. Economic aspects were explored by 8/10 HTA bodies; organisational issues by 7/10; technical and social domains by 5/10; ethics by 4/10; and environmental issues by 2/10 (Table 2). Most of the responses received did not include an assessment of areas not covered by the EUnetHTA core model, specific to digital aspects such as: technical stability, interoperability, ease of use and accessibility, security and data protection. Several countries have taken these aspects into account, either directly in the HTA assessment or as a prerequisite.
Table 2 Evidence characteristics and requirement analysing the survey responses from 9 EU countries HTAs and one non-EU
Regarding evidence requirements (Table 3), the six responses available were in complete agreement on the need for further clinical studies. Five out of six respondents answered questions about eligible types of studies. Some HTA bodies required randomized clinical trials (RCTs). These RCTs could be associated with different assumptions such as superiority or non-inferiority. For example, one HTA body (Germany) did not accept single arm studies while four HTA bodies accepted these. The responses did underline that for DMDs, new types of clinical study designs could be accepted, such as adaptive interventional studies or real-world evidence.
Table 3 Clinical evidence framework and clinical study type analysing the survey responses from 9 EU countries HTAs
There was general consensus on the acceptance of clinical evidence data collected in other EU countries. Nevertheless, some agencies (i.e., France’s Haute Autorité de Santé – HAS – and Portugal’s Autorida de Nacional do Medicamento e Produtos de Saúde I.P. – INFARMED) required similarity of healthcare contexts. Others (i.e., Germany’s Bundesinstitut für Arzneimittel und Medizinprodukte – BfArM) emphasised their preference for national data, even though foreign clinical data could be accepted in some cases.
Description, usage and interpretation of the Evidence Matrix
The Evidence Matrix presented in Table 4 is based on the scope of DMDs defined for the European classification grid as well as input received through the survey and the workshop. The matrix is divided in the following fourmain sections: (a) “usage” – use of the DMD as stand-alone device and/or as part of a recognised integrated care pathway, (b) CE marking – risk classification (I, IIA, IIb, III), (c) “regulatory setting landscape” – DMD falling under HTA evaluation policy, (d) “DMD evidence core model”- evidence domains for assessment: CUR (Current use), TEC (Technical), TEC AI (Technical for AI based DMDs), PERF (Performance), SAF (Safety), EFF (Efficacy), ECO (Economics), ORG (Organisational), ETH (Ethics), SOC (Social), SEC, (Security) INT (Interoperability).
Table 4 Description of DMD evidence core model associated with the Evidence Matrix
The domains are based on EUnetHTA’s HTA Core Model12, (i.e., CUR-TEC-ORG, EFF-SAF-ECO and SOC-ETH) to which the following domains are added: TEC-AI, PERF, SEC and INT. Each of these items is detailed and described in Table 5. The HTA Core Model domains need to be interpreted and applied to the DMD context. The additional novel domains are added based on the findings in the literature, the answers to the survey and the evidence generation workshop, to ensure the framework would fit all device types13, their respective functionalities and capacity to respond to specificities of DMDs.
Table 5 Evidence Matrix to be associated with the CEUGrid-DMD
It is important to highlight that these DMD domains should be read and interpreted considering not only the software itself but also how their use will be integrated into the patient care pathway and in the overall health care system.