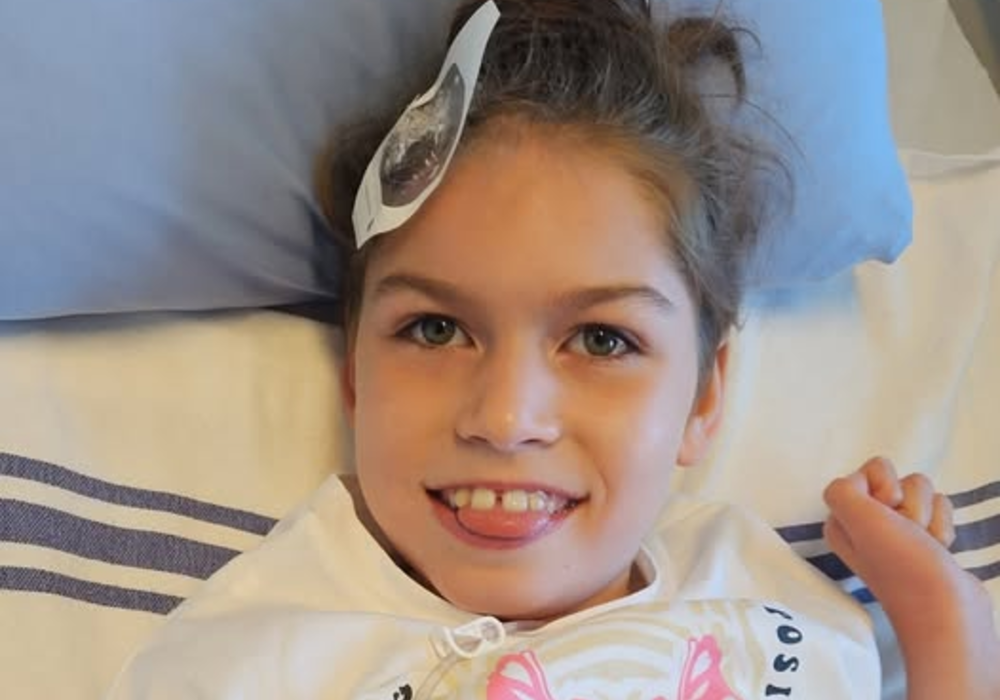
A family in Langford has been left devastated after the BC government has denied further coverage for an expensive drug that has been critical to sustaining their child’s life.
Charleigh Pollock, who is just 9-years-old, is the only child in BC with a diagnosis of neuronal ceroid lipofuscinosis type 2 (CLN2), or Batten Disease.
This disease is extremely rare, impacting only 0.15 to 9.0 children per 100,000 live births.
It is a terminal diagnosis and causes seizures, vision loss, problems with thinking and movement as well as a shortened life expectancy—those with Batten may only live to be between 7 and 10-years-old.
However, with the drug cerliponase alfa (Brineura), children with Batten Disease are offered a lifeline and often live into adolescence.
Currently, there is no cure for Batten Disease and Brineura is the only option for treatment.
In the case of Charleigh, her mother says that the drug has been extremely effective.
“We’ve gone from almost a hundred seizures a day down to none,” said Jori Fales, Charleigh’s mom. “We see the improved quality of life, we see how much this drug has slowed her disease progression.”
“This isn’t just a drug—it’s her lifeline…her only defense against this disease.”
However, Charleigh will now have to go without her only lifeline, as she is no longer approved to have the drug.
Victoria Buzz received a statement from Josie Osborne, Minister of Health in BC, regarding Charleigh’s case and the reasoning for cutting her off from the drug.
“I empathize deeply with Charleigh’s family, and my heart goes out to them,” said Osborne.
“During my time as Health Minister, this has been one of the most challenging files my team and I have worked on.”
She continued that clinical evidence shows that once a patient passes a threshold of decline in their motor and language skills, Brineura no longer works to slow the progression of Batten Disease.
“Last year, it was determined that Charleigh’s condition had progressed to the point where she met the ‘discontinuation’ criteria for Brineura,” stated Osborne.
A recent review of Brineura was conducted by the Canadian Dug Agency (CDA) which found that the drug “improved quality of life (QoL), the mitigation of disease progression, and patient safety.”
However, the review found that evidence is limited when it comes to advanced cases of the disease and when to stop treatment.
This controversial decision has been criticized by Brennan Day, MLA for Courtenay-Comox and Opposition Critic for Rural and Seniors’ Health.
“It has become increasingly apparent that no Batten disease specialists were consulted as part of this decision,” said Day.
“We have yet to see any evidence that international or Canadian subject matter experts were brought in to assess Charleigh’s case or advise the provincial review board.”
Day added that this situation isn’t just about policy, “it’s about science, compassion, and doing the right thing.”
Charleigh’s mom says that her medical team disagrees with the Province’s decision to remove her from accessing the drug, given that it has helped her quality of life, her seizures have been drastically reduced and her language and motor skills have been preserved far longer than they would have been.
“It is NOT Charleigh’s time to discontinue Brineura. She is still benefiting, and we implore the province to allow her to continue living and accessing this drug,” said Fales.
“Anything less is simply cruel and wrong.”
Despite Charleigh’s family’s hopes for a different decision, Osborne and the Province are standing by the life-altering choice to cease her access to the drug.