In the following, we used heat flows to accumulate and confine a functioning cytoplasmic mimic from a concentration of components that was too low to support activity (Fig. 1). To that end, we used TX-TL (PURExpress) as a model of a minimal cytoplasm of a living cell. PURExpress is a commercially available version of the PURE system41 and consists of the purified Escherichia coli translation machinery, T7 RNA polymerase, an adenosine triphosphate (ATP) regeneration system, ribonucleotide tri-phosphates, transfer RNAs (tRNAs), amino acids, salts and cofactors.
Fig. 1: Thermally assembled protocell.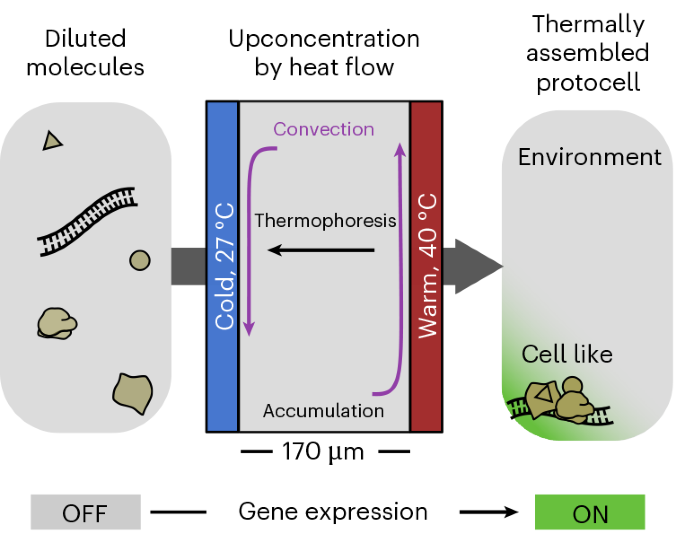
A microscale reaction chamber was filled with the components of a recombinant in vitro transcription–translation system at a concentration that was too low to support biological activity. The application of a temperature difference triggered thermophoresis and convection inside the solution. The combination of both effects concentrated the TX-TL components towards the bottom of the chamber and, thus, activated the gene expression (green area).
We developed custom-made chambers that allowed the establishment of a well-controlled temperature difference across a gap of 170 µm and permitted optical access for real-time fluorescence imaging under conditions optimized for low protein adsorption33,34 (Supplementary Figs. 1 and 2). To confirm the compatibility of the setup with cell-free protein synthesis, we supplemented the TX-TL solution with a linear DNA template encoding superfolder green fluorescent protein (sfGFP)42. As expected, we observed a homogeneous, time-dependent increase in green fluorescence over the whole chamber on incubation at 37 °C (Supplementary Fig. 3).
Thermal accumulation as the basis for protocell assembly
After having confirmed the compatibility of TX-TL with the setup of the thermophoretic chamber, we next sought to explore the accumulation behaviour of the system within an externally applied thermal gradient. Two processes dominate the motion of solute molecules on the application of a temperature difference at the microscale. First, molecules consistently move along the temperature differences in our settings from the warm to the cold side. This effect is termed thermophoresis or thermal diffusion. Second, the density difference in the differentially heated solution creates a laminar flow, which shuttles the fluid downwards on the denser, colder side. At moderate convection speeds, solute accumulation is enhanced by the superposition of both effects. The combined effect is termed thermogravitational or thermal accumulation and has been recently experimentally and computationally demonstrated to be capable of accumulating biomolecules in simple molecular solutions32,34,37,40.
To characterize the thermophoretic properties of our reporter molecule sfGFP in the viscous environment of the TX-TL solution, we initially conducted a numerical model using finite element methods (the ‘Material and methods’ section in Supplementary Information). The model accounted for heat conduction, laminar fluid dynamics, gravity, thermophoresis and diffusion. Using this model, we found that a chamber thickness of 170 µm leads to an optimal convection speed that maximizes the accumulation of sfGFP within our experimental timescale (Supplementary Fig. 4a). The experimental data fitted best to a model in which the viscosity of the TX-TL solution was 1.3-fold higher than water (see the ‘Material and methods’ section in Supplementary Information).
Next, we ascertained whether the thermophoretic accumulation of sfGFP was possible within the highly complex molecular mixture of a TX-TL system. We initially expressed sfGFP at 37 °C for 4 h until reaching the plateau phase and then applied a temperature difference between 27 °C and 40 °C. This triggered the accumulation of sfGFP, which reached an accumulation steady state after about 24 h (Supplementary Fig. 5a and Fig. 2a (solid lines)). Fluorescence microscopy revealed an ~3-fold sfGFP accumulation at the bottom of the chamber with respect to the fluorescence level obtained at the plateau phase of protein synthesis before applying the temperature difference. The fluorescence at the top was instead depleted (solid lines). The experimental fluorescence data were fitted by the finite element model to estimate the thermophoretic mobility of sfGFP within the complex TX-TL solution (Fig. 2a (dotted lines); see the ‘Material and methods’ section in Supplementary Information).
Fig. 2: Compartmentalization and feeding by a temperature difference.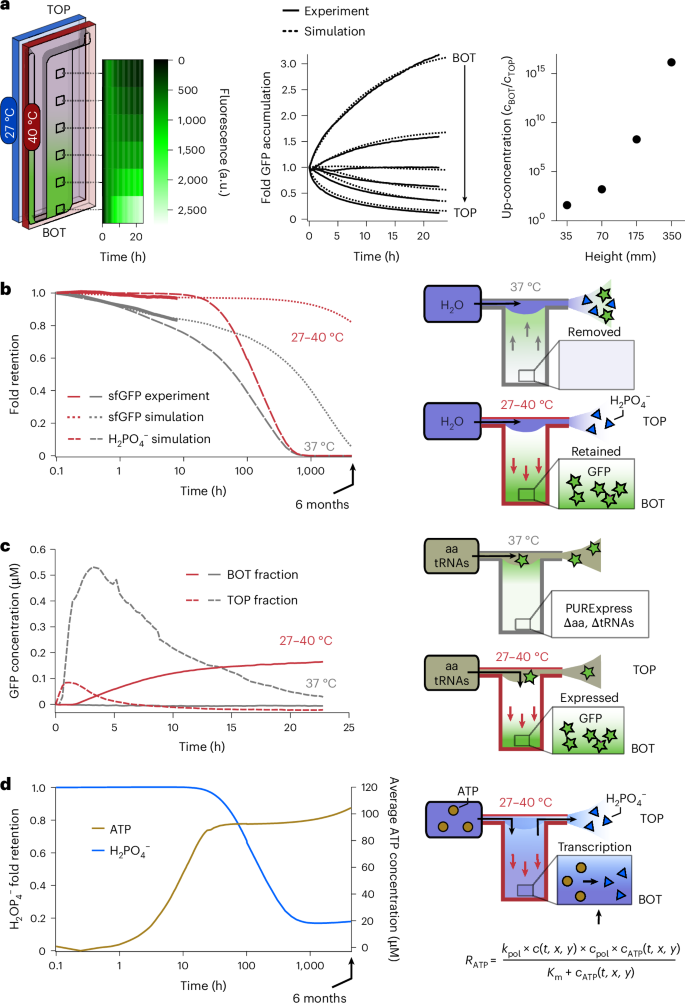
a, A thermophoretic chamber filled with a TX-TL solution, expressing sfGFP at the steady state, was subjected to a temperature difference (27–40 °C). The accumulation of sfGFP was monitored by measuring fluorescence over time at different chamber positions (black squares), recording a 3-fold absolute and 25-fold relative accumulation (solid lines). The dashed lines correspond to a simulated finite element model (see the ‘Material and methods’ section in Supplementary Information). b, The setting was challenged by a continuous flow of water at the top of the chamber (11 µl h–1; dark blue) to test the thermal confinement of sfGFP provided by the temperature difference. Without the temperature difference, diffusion into the outward flow slowly removed sfGFP from the chamber (grey arrows). The total pre-expressed sfGFP fluorescence remaining inside the chamber was modelled by finite element simulations for up to 6 months (dotted lines). The solid lines are averages of three experiments. As a comparison, \({{\rm{H}}}_{2}{{\rm{PO}}}_{4}^{-}\) was simulated as a possible waste molecule produced during sfGFP synthesis (large, dotted lines). \({{\rm{H}}}_{2}{{\rm{PO}}}_{4}^{-}\) was removed much faster from the thermal trap than sfGFP under isothermal conditions but also when a temperature gradient was present. c, Activation and retention of a deficient TX-TL reaction was probed by supplying the missing amino acids and tRNAs at a flow rate of 11 µl h–1 at the top of the chamber. The sfGFP concentration was measured at the top and bottom of the chamber with and without applying a temperature difference. In isothermal conditions, the TX-TL reaction was first activated and then flushed out, leaving no synthesized sfGFP in the compartment in the long run. However, when a temperature difference was applied, the reaction was activated by the accumulated supply solution, leading to the synthesis and long-term accumulation and retention of sfGFP. d, Simulated kinetics of ATP (brown) and \({{\rm{H}}}_{2}{{\rm{PO}}}_{4}^{-}\) (blue) as feeding and waste molecules, respectively, in a simulated transcription reaction under an active thermal gradient. ATP was introduced in the trap at a concentration of 1 mM under the same fluid flow as in previous experiments and simulations. \({{\rm{H}}}_{2}{{\rm{PO}}}_{4}^{-}\), which is present in the trap before the start of the flow, is simultaneously removed from the trap by the continuous fluid flow at the top of the chamber and produced from ATP throughout the trap corresponding to the concentration of the TX-TL reaction. A stable state is reached after 800 h, in which \({{\rm{H}}}_{2}{{\rm{PO}}}_{4}^{-}\) is produced at the same rate as it is removed. On the other hand, ATP is continuously accumulating in the chamber and retained against the fluid flow.
Importantly, we found similar sfGFP fluorescence levels when the temperature difference was applied from the start of the experiment (Supplementary Fig. 5b,c), indicating simultaneous accumulation and gene expression. The maximum temperature difference that we could use for the accumulation experiments was found by the isothermal expression of sfGFP in a test tube. sfGFP was expressed from 27 °C to 42 °C, showing a maximum protein yield in the range of 33–39 °C (Supplementary Fig. 6), in agreement with a previous report25.
These results indicated that even without an active membrane compartment, two spatially distinct regions formed within the differentially heated chamber, consisting of a depleted region at the top and a region at the bottom that was enriched in sfGFP. We, therefore, hypothesized that the upper region could form an extracellular space, whereas the bottom region could give rise to a confined, functional mimic of a crowded cytoplasm.
Thermally induced confinement
The cytoplasm of a cell is confined and separated from the environment by a semipermeable membrane. Cell membranes control the exchange of molecules with their surroundings and help tolerate different stress conditions. For example, endothelial cells regulate the molecular exchange between the bloodstream and neighbouring tissues. They do so without being disrupted or diluted by the blood flow and actively maintain their cytosolic components at working concentrations43.
To determine if a temperature difference could give rise to similar properties, we opened the top part of the chamber and provided a constant inflow and outflow of water at a rate of 11 µl h–1, 22 µl h–1 and 36 µl h–1 (Fig. 2b and Supplementary Fig. 7). In this way, we could assess whether the expressed and accumulated sfGFP could be confined and protected against dilution through a temperature difference rather than a semipermeable membrane.
To this end, TX-TL solutions were incubated with or without a temperature difference over 16 h to express and accumulate or only express sfGFP, respectively. Subsequently, the chambers were exposed to a flow of water at a rate of 11 µl h–1 for 9 h. A numerical model (Supplementary Fig. 8 and the ‘Material and methods’ section in Supplementary Information) predicted a near-complete removal of sfGFP after 6 months for the chamber incubated isothermally, in agreement with the experimentally recorded initial trend (Fig. 2b, grey lines). This result was expected by diffusion from the geometry features of the chamber with a height of 35 mm. Conversely, for the chamber subjected to a temperature difference, the model predicted a 97% retention of the sfGFP molecules after 9 h and still 83% after 6 months (Fig. 2b, red lines), even when the top of the chamber was continuously diluted by the water flow. The removal of small molecules in the chamber was simulated under the same conditions using orthophosphate (\({{\rm{H}}}_{2}{{\rm{PO}}}_{4}^{-}\)) as a waste molecule44 since the diffusion and Soret coefficients were known (959 µm2 s–1 and 0.7 × 10–3 K–1 (ref. 34), respectively). Phosphate is a byproduct of many processes in the TX-TL reaction including RNA polymerization. The inorganic phosphate competes with energy molecules such as ATP and guanosine triphosphate for Mg2+ ions and inhibits transcription and translation by sequestering them. \({{\rm{H}}}_{2}{{\rm{PO}}}_{4}^{-}\) was removed notably faster from the chamber under isothermal conditions compared with sfGFP owing to the lower diffusion coefficient of the latter. Furthermore, if a thermal gradient was applied, sfGFP remained trapped and fast-diffusing phosphate was removed with a delayed onset (Fig. 2b).
Next, we investigated whether the TX-TL reaction could be simultaneously activated and confined (Fig. 2c). For this purpose, the chamber was prefilled with a TX-TL solution without amino acids and tRNAs, and thus unable to synthesize proteins. To probe for protein synthesis, amino acids and tRNAs were continuously supplied from the top of the chamber at a rate of 11 µl h–1 for 22 h. Under isothermal conditions (37 °C), the sfGFP concentration quickly rose at the top where the feeding solution entered the chamber, reaching a maximum of 0.53 µM, whereas no expression was observed at the bottom of the chamber due to limited diffusion of the feedstock molecules. However, this left the product at the upper chamber highly susceptible to the outward-directed flow, leading to a steady drop in the synthesized sfGFP concentration. By contrast, applying heat flows under otherwise identical conditions resulted in a strong sfGFP fluorescence signal at the bottom of the chamber, indicating its expression protected from the harmful flow-through at the top. The steadily growing sfGFP concentration reached a value of approximately 0.18 µM by the end of the experiment. Therefore, we concluded that a temperature difference can robustly confine sfGFP for extended periods of time. As a result, the upper part of the chamber acts in a similar way as a diluted extracellular fluid, whereas the lower part resembles a crowded cytosol-like compartment capable of using feedstock molecules from the environment.
Using the model developed with retention, a possible feeding mechanism was simulated. ATP was introduced into the chamber at a concentration of 1 mM carried by the same fluid flow as in previous simulations. In the chamber, ATP was continuously accumulated and converted to \({{\rm{H}}}_{2}{{\rm{PO}}}_{4}^{-}\) according to a Michaelis–Menten kinetic equation45 simulating, for example, a transcription reaction. The results of the simulation showed that the \({{\rm{H}}}_{2}{{\rm{PO}}}_{4}^{-}\) present in the chamber before the start of the fluid flow is gradually removed from the trap, after the flow has been started, much faster than the background production (Fig. 2d, blue line). After approximately 800 h, the \({{\rm{H}}}_{2}{{\rm{PO}}}_{4}^{-}\) concentration reaches a steady state in which it is produced and removed from the chamber at similar rates. By contrast, ATP is introduced and retained in the thermal trap, leading to a steady increase in concentration even as it is continuously consumed by the reaction (Fig. 2d, brown line).
Thermally induced cellular activity
Living cells, as well as in vitro TX-TL systems, require optimized concentrations of their molecular constituents to operate efficiently14,15. Cells actively maintain the working concentration of their components by means of chemical energy (ATP) and membrane transport proteins. We next tested whether a similar process could be solely driven by a temperature difference. To that end, we assessed (1) whether all the components of a TX-TL system could be co-accumulated by a temperature difference and (2) whether the accumulation would be strong enough to activate transcription and translation reactions starting from a dilute, inactive TX-TL system. Normally, the TX-TL reaction has an inherent robustness against slight changes in the concentrations of its components. However, drastic changes in composition usually lead to the inactivation of the reaction46,47.
The accumulation experiment shown in Fig. 2a gave us an initial indication of the relative and absolute accumulation of sfGFP in our experimental setup. We found a 25-fold relative accumulation, calculated as the ratio of sfGFP fluorescence between the bottom and top of the chamber (BOT/TOP). This value specified the magnitude of the concentration imbalance created by the temperature difference along the height (longitudinal axis) of the chamber. By contrast, we detected a 3-fold absolute accumulation of sfGFP at the bottom of the chamber. This value corresponds to the ratio of sfGFP fluorescence between the end time point and the start of the experiment (BOT_final/BOT_start) and indicates the enrichment of protein at the bottom of the chambers with respect to the initial state.
On the basis of these results, we wondered whether all the other components of a TX-TL system could be similarly accumulated under the same conditions. To address this, we probed the accumulation of ions, amino acids, ribonucleotides, DNA and proteins by measuring their relative and absolute accumulations, starting from one-third of their working concentration inside a closed chamber.
After 16 h of incubation, we froze the chamber content and sectioned the frozen samples into three equal parts (top, middle and bottom), as detailed and tested in a previous study40. Then, we analysed each section by gel electrophoresis and chromatography (Fig. 3a). Bands from agarose and denaturing sodium dodecyl sulfate polyacrylamide gels corresponded to the average amount of DNA and protein from each of the three sections of the chamber.
Fig. 3: Temperature difference triggers cellular activity.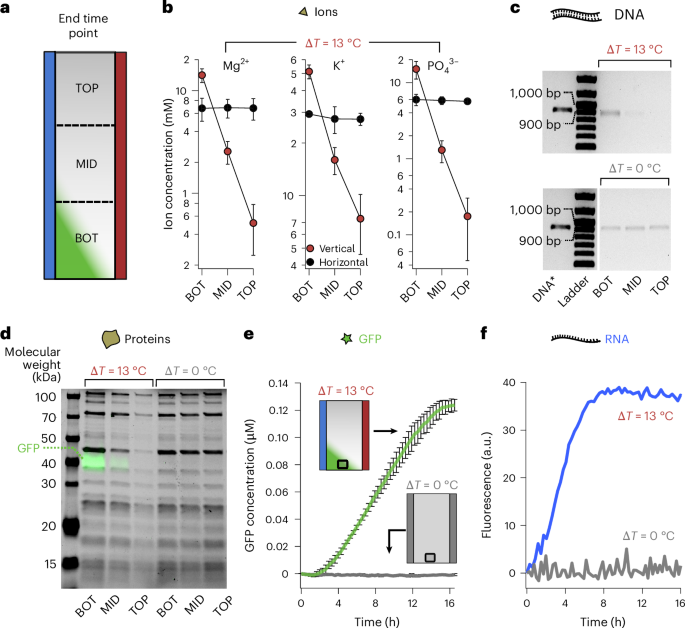
a, Schematic of a thermophoretic chamber subjected to a temperature difference (27–40 °C). After 16 h of incubation, the top (TOP), middle (MID) and bottom (BOT) fractions were freeze extracted and sampled for analysis. Chamber controls devoid of accumulation were incubated isothermally (ΔT = 0 °C) to switch off thermophoresis or rotated 90° into a horizontal orientation with (ΔT = 13 °C) to stop thermal convection. b, Ion concentration was probed by chromatography. Mg2+, K+ and \({{\rm{PO}}}_{4}^{3-}\) showed a strong thermophoretic response with 30-, 7- and 85-fold relative accumulation and 2.5-, 2- and 2.7-fold absolute up-concentration at the bottom of the chamber. The data are represented as the mean value of triplicate experiments ± standard deviation. c, Agarose gel shows a 2.7-fold absolute accumulation of the linear DNA template encoding sfGFP used for the experiments. DNA* is the linear DNA fragment as a migration reference. d, Merged image of a sodium dodecyl sulfate polyacrylamide gel. The black bands are PURExpress proteins visualized with stain-free technology (Bio-Rad). The relative and absolute accumulations of the total protein were, on average, 6.4- and 1.8-fold, respectively. The green bands correspond to the intrinsic fluorescence of sfGFP. e, sfGFP fluorescence kinetics measured at the bottom of the chambers (black squares) from 3-fold-diluted TX-TL reactions incubated with and without a temperature difference (green and grey, respectively). Gene expression was only observed when the temperature difference was applied. The data are represented as the mean value of triplicate experiments ± standard deviation. f, Transcription from DNA to RNA was separately visualized using a PCR fragment coding for F30 Broccoli aptamer, showing RNA concentration by fluorescence. The observed accumulation rose faster, probably due to faster transcription, but also from a superior thermal accumulation of RNA at the bottom of the chamber. No signal could be detected in isothermal conditions, suggesting no active transcription.
Ions involved in transcription and translation48,49 showed a strong thermophoretic response. Relative accumulations were 30-fold for Mg2+, 7-fold for K+ and 70-fold for \({{\rm{PO}}}_{4}^{3-}\) (Fig. 3b). The observed variation in relative accumulation was due to the charge and size dependencies of ionic thermophoresis50. At the bottom of the chambers, 2.5-, 2- and 2.7-fold absolute accumulations were obtained, respectively, for the same ions, averaged over the extracted bottom section (Supplementary Fig. 9a).
Comparable absolute fold accumulations were obtained for amino acids and ribonucleotides (Supplementary Table 1) and for DNA, where a 2.7-fold absolute accumulation was obtained for the bottom fraction (Supplementary Fig. 9b). The relative accumulation of DNA was estimated to be larger than 40-fold as the depletion at the top was close to the background DNA signal levels (Fig. 3c).
The protein components of the TX-TL system, which considerably differ in molecular weight, showed coherent accumulation patterns as measured by single-band quantification from polyacrylamide gels with 2.4- to 23-fold relative accumulations resulting in an average value of 8.6-fold (Fig. 3d and Supplementary Fig. 9c). The absolute accumulation at the bottom fraction ranged from 1.3-fold to 2.3-fold with an average value of 1.9-fold (Supplementary Fig. 9c). In general, the thermophoresis of proteins is known to be comparably small51. Moreover, the similar accumulation patterns of most TX-TL proteins might also reflect their co-localization during coupled transcription and translation reactions including more than 50 ribosomal proteins that are integral parts of the 50S and 30S ribosomal subunits.
Overall, the concentration of ions, amino acids, ribonucleotides, DNA and proteins followed an exponential distribution along the chamber height. Therefore, the local concentration at the very bottom of the chamber was notably higher than that observed experimentally, which corresponded to the average value obtained over 33% of the chamber volume. As expected, non-accumulating isothermal or horizontally oriented control chambers did not show an effective accumulation of TX-TL components (Fig. 3b,d and Supplementary Fig. 9).
Finally, we assessed whether similar levels of ion, amino acid, ribonucleotide, DNA and protein accumulations could lead to the synergistic transcription–translation activation and, therefore, sfGFP synthesis. We started from a TX-TL solution with a 3-fold lower concentration of its components. At such a reduced concentration, no detectable sfGFP expression was found when incubated isothermally at 37 °C (Fig. 3e (grey), Supplementary Fig. 10a and Supplementary Video 2), in agreement with a previous report14. The fluorescent protein was neither detected at 27 °C nor 40 °C (Supplementary Fig. 11). Intriguingly, the application of a temperature gradient led to a strong expression of sfGFP at the bottom of the chambers (Fig. 3e (green) and Supplementary Video 1) by the combined co-accumulation of TX-TL components. Furthermore, as expected with the observed accumulation of ribonucleotides (Supplementary Table 1), the recovery of transcription from a similarly diluted TX-TL reaction was confirmed separately with the Broccoli aptamer52 (Fig. 3f, green).
The thermogravitational accumulation dependence on chamber thickness was experimentally confirmed by measuring the protein accumulation and sfGFP synthesis along the height of the thermophoretic chambers with different thicknesses (Supplementary Fig. 4b,c). As predicted by the model, we found only minor synthesis of sfGFP for a thinner 125-µm chamber, whereas at 250 µm, no accumulation of proteins and, therefore, no sfGFP synthesis could be observed.
Time-resolved gel and chromatography analyses revealed that DNA and protein accumulations preceded the onset of sfGFP synthesis for several hours, suggesting that the accumulation kinetics of small-molecular-weight components such as ions and amino acids are rate limiting for the initiation of protein synthesis (Supplementary Fig. 12).
In addition, to test the limits of what can be assembled/accumulated with thermogravitational accumulation, we attempted to recover the synthesis of sfGFP from a more complex molecular mixture such as the one provided by a cell-free extract (Supplementary Fig. 13). In this case, the proteins did not accumulate substantially at the bottom fraction of the chamber, possibly due to a higher viscosity of the cell-free extract. sfGFP, however, was present more abundantly at the bottom, decreasing in intensity non-exponentially to the top of the chamber, in contrast to model expectations. This suggests that the reaction was active, but the accumulation mechanisms were markedly more complex. To further clarify the relationship between the accumulation of TX-TL components and sfGFP synthesis, 3-fold-diluted reactions were mixed with glycerol to a final concentration of 10% or 20% (v/v). The solutions were incubated under the same conditions as in the previous experiments. In both cases, the onset of sfGFP synthesis was delayed compared with the control experiment (Supplementary Fig. 16), probably due to slower convection currents. Interestingly, higher levels of sfGFP fluorescence were detected at the bottom of the chamber for 20% glycerol compared with 10%, but both were below the levels without added glycerol. Since the solutions did not reach the plateau phase during the 18-h incubation period, it is possible that the overall sfGFP yield is higher than in standard reactions.
Furthermore, to better understand the kinetics of the system, additional experiments were performed in which, after an initial 18-h incubation period in a thermal gradient and sfGFP synthesis, the gradient was turned off and the chamber was isothermally incubated at either 27 °C or 40 °C. The decrease in concentration at the bottom of the thermal trap due to sfGFP diffusion was observed for another 6 h. As expected, diffusion was much faster at 40 °C than at 27 °C, as indicated by the rate at which the fluorescence intensity decreased compared with the beginning of the isothermal incubation period (Supplementary Fig. 17). Additionally, we simulated the isothermal conditions at 27 °C for a longer period (10,000 h) and found that a uniform distribution of sfGFP is reached after approximately 3,000 h. This highlights the longevity of accumulation by thermophoresis.
Interestingly, it was not necessary to tune the individual initial TX-TL component concentrations to obtain an operational TX-TL system since the strength of thermophoretic accumulation is known to be independent of the starting molecular concentrations33,34,50,53 and led to similar absolute fold accumulations. The coordinated accumulation achieved a sufficiently high concentration of all the molecular components to activate protein synthesis. Protein concentration calibrations in custom-made PURE showed that the overaccumulation of individual proteins still resulted in a functional TX-TL reaction, but without increasing the protein yield54, demonstrating a robustness that probably helped our experiments. Within our experimental setting, the recovery of sfGFP was observed up to a 4-fold-diluted TX-TL reaction. Higher dilutions led to no measurable signal (Supplementary Fig. 10b).
Thermal protocells potential and implications
Overall, our results showed that the heat-flow-driven accumulation of ions, amino acids, ribonucleotides, DNA and proteins led to a confined functional gene expression system at the bottom of the compartment, insulated by the absolute thermophoretic accumulation from the top of the chamber. Consequently, the prebiotic period in which this accumulation mechanism would be the most relevant would be from the formation of the first biomolecules to the encapsulation of these biomolecules in lipid vesicles. At this stage, dilution should not be as much of a problem for the nascent prebiotic networks. Furthermore, thermogravitational accumulation may even aid encapsulation by accumulating lipid precursors above the critical vesicle concentration36. In our fluorescence images, we could not find evidence for coacervate formation or a liquid–liquid phase separation16. The molecules are not accumulated to the extent that a phase transition is triggered, and we rather observe cooperative concentration enhancement in the PURE expression system by the thermophoretic forces.
In future experiments, higher thermophoretic chambers are likely to overcome higher dilutions of the TX-TL reaction. However, one limitation not related to the accumulation strength of the thermogravitational mechanism is the dissociation of ribosomes at high dilutions. The E. coli ribosomes used in the TX-TL reaction are highly dependent on the Mg2+ concentration. The more the reaction is diluted, the greater the disassembly of the ribosomal subunits, to a point where reversibility by accumulation is also limited55,56. It should also be noted that the absolute accumulation of molecules was limited in a closed chamber. Further geometrical optimizations would need to be undertaken to confine and maintain the activity of the TX-TL reaction for increasingly longer periods of time by supplying nutrients to the reaction and removing waste molecules. Such an open system with an external supply of TX-TL components and continuous accumulation could achieve a substantially higher absolute concentration, potentially reaching values known from biological cells57. Additionally, the temperature difference offers dynamic and direct access to the inner concentrations of the components of the thermally assembled protocell, enabling multiple modes for molecular exchange including feedback, signalling, feeding and chemical communication.