Source of information
All data related to NF applications were extracted from publicly available information on the EFSA website13, with data collection completed on October 4, 2024, and from the corresponding published EFSA scientific opinions on NFs. The analysis includes only applications submitted under the current Regulation (EU) 2015/2283, which came into effect on January 1, 2018. Applications submitted before this date were not considered in this analysis. Only applications specifically related to NFs authorization were considered, excluding those withdrawn by applicants, terminated by EFSA, or concerning traditional foods from third countries.
Information analyzed
Applications were classified into four categories based on their status on October 4, 20241: intake (suitability check by EFSA is ongoing)2, ongoing risk assessment (scientific evaluation has started), and3 adopted (the evaluation is finalized and the opinion has been adopted, this category includes applications marked as ‘publishing’ or ‘published’ on EFSA platform), and4 not valid (application rejected by EFSA, in most of the case due to non-compliance with the Transparency Regulation), as presented in Table 1.
Table 1 Status of applications according to EFSA website on October 4th, 2024
Key information extracted for analysis includes: the date of submission of the application to the EC, the date the dossier was received by EFSA, the dossier validation date (the date on which the EFSA suitability check is completed, and validity of the application is confirmed by EC, marking the start of the scientific evaluation), the date of rejection of the application (if applicable), the number and dates of ADRs issued by EFSA, the dates the applicant responded to these ADRs, the date EFSA adopted its opinion, and the date of publication of the opinion. The date of submission of the application and the dates of the ADRs were extracted manually from the published EFSA scientific opinions. The rejection date for applications deemed not valid was extracted manually from the EFSA website13. The dossier received date, dossier validated date, opinion adoption date and publication date were extracted automatically from the EFSA website13.
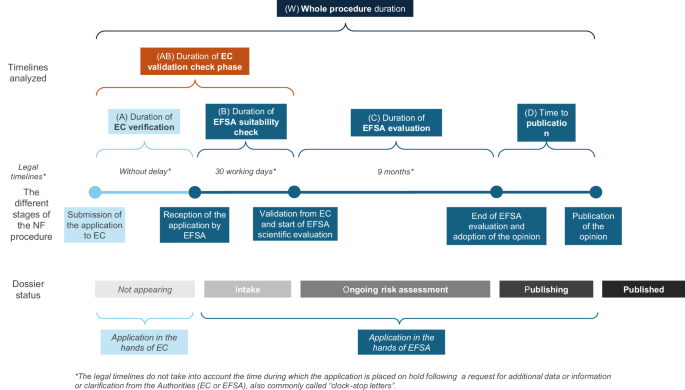
Based on these data points, several timelines were calculated to provide a comprehensive overview of the process (see Table 2 for the definition of each calculated timeline). These calculations include (A) the time taken by the EC to verify the application, (B) the time taken by EFSA to check the suitability and completeness of the application, (AB) the total duration of the validation phase by EC and EFSA (i.e. the time from submission to the start of the scientific evaluation), (C) the duration of the scientific evaluation by EFSA (measured from the validation date to the adoption of EFSA’s opinion), (D) the time from the adoption of the opinion to its publication, and (W) the overall duration of the process encompassing all stages (A, B, C, D) from submission to publication. Figure 1 provides a graphical representation of the different stages of the NF authorization process, and the timelines calculated.
The different stages of the NF procedure and the calculated timelines.
Table 2 Definition of the various timelines calculated based on EFSA data
Only applications classified as2 ongoing risk assessment3, published, and4 not valid were included in the timeline analysis. No timeline could be calculated for applications in category1 intake, as only the date of receipt by EFSA was available.
For applications rejected by EFSA due to non-compliance with Article 32b of the Transparency Regulation (Regulation (EU) 2019/1381), the (E) timeline, defined as the time from EFSA’s receipt of the application to the date of rejection, was also calculated.
Finally, the final outcome (i.e., positive or negative) of the published opinions was analyzed. Data are reported as mean ± standard deviation.
Timeline of novel food applicationsStatistics of the applications on October 4th
Table 1 presents the number of applications evaluated in this analysis (n = 292), their status on the EFSA platform as of October 4th, 2024, and the category to which they were assigned. Among these, 62 applications were under suitability check (i.e., intake), 107 were undergoing scientific risk assessment, 93 were adopted (i.e., published or publishing), and 30 were rejected as not valid.
Figure 2 shows the number of applications submitted per year, categorized by their assigned status as of October 4th, 2024. Overall, 36 applications were submitted in 2018, 40 in 2019, 43 in 2020, 55 in 2021, 39 in 2022, 34 in 2023, and 41 in 2024. The submission date was not available for 4 applications currently under evaluation.
Fig. 2: Status on October 4th, 2024, of the 292 applications evaluated per year of submission.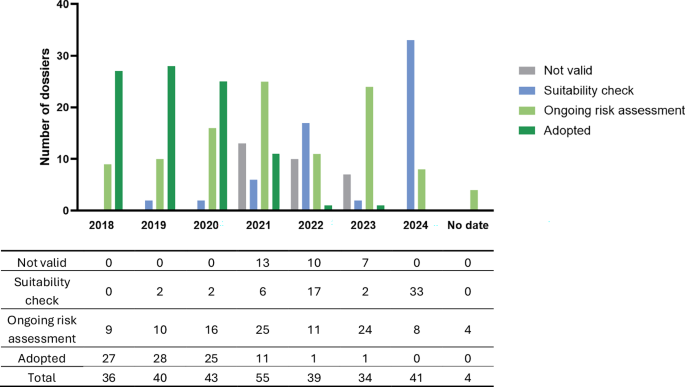
The date of submission is not available for four applications currently under EFSA evaluation. For some dossiers, the submission year was not available, the submission date was then estimated based on the EFSA question number and NF dossier number.
Duration of the validation check phase by the European Commission (AB)
A total of 200 applications submitted after 2018 were considered valid by the EC and EFSA, and are now either under scientific evaluation (n = 107) or have been finalized (n = 93). Among these applications, data was not available for 4 applications, while two applications related to insects were identified as outlier due to abnormally long EC validation times, and were excluded from the analysis (applications NF 2018/0395 and NF 2018/0128, 1620 and 2296 days for validation respectively). Consequently, data from 194 applications were included in the analysis.
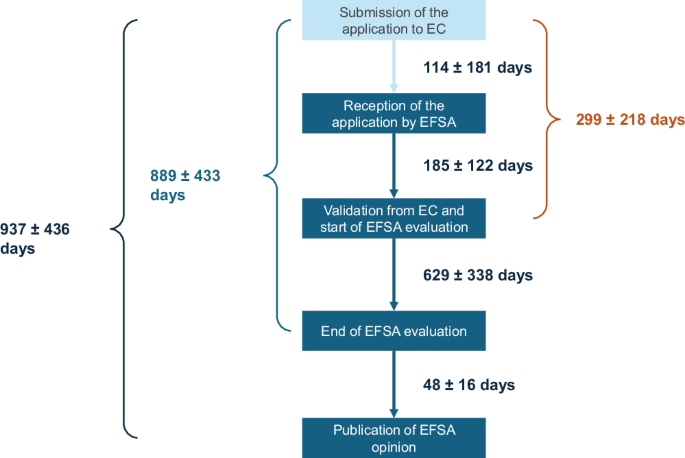
The average time needed for the EC to verify the application after submission is (A) 114 ± 181 days (range: 0–1430 days). The mean length of EFSA suitability check is (B) 185 ± 122 days (range 15–758 days). The average duration of the whole validation check phase, defined as the total time between the submission of the application and the start of its evaluation, is (AB) 299 ± 218 days (range 20–1635 days).
Duration of the scientific evaluation of the applications by EFSA (C)
EFSA has published or is publishing 93 NF opinions for applications submitted since 2018. However, the date of validation is not available for 5 applications. For the remaining 88 opinions, average duration of EFSA evaluation was (C) 629 ± 338 days (i.e. 20.7 ± 11.1 months). The shortest evaluation lasted 179 days (i.e. 5.9 months), while the longest one lasted 1714 days (i.e. 56.4 months).
The average number of ADRs from EFSA is 2.7 ± 1.9, ranging from 0 to 8 requests. Applicants need 130 days on average to answer one EFSA ADR (range 0–733 days). The overall cumulated time needed to answer all EFSA ADRs is 353 ± 299 days (range 0–1213 days). The time taken by applicants to answer EFSA requests represents 47 ± 25% of the total evaluation time, while EFSA evaluation represents only 53 ± 25% of the total time needed for evaluation.
Among these 93 applications published or publishing, EFSA exceeded the standard nine-month evaluation period for 24 applications, representing 26% of the applications. The average overrun is 156 ± 212 days (range: 5–951 days). It should be noted, however, that Article 22 of Regulation (EU) 2015/2283 authorizes the EC to extend the time period for evaluation.
Time to publication (D)
For the 91 published EFSA outputs, the average time from the vote of the Nutrition, Novel Foods, and Food Allergens Panel to the publication of the opinion (D) was 48 ± 16 days, with a range of 26 to 107 days.
Among these 91 EFSA opinions evaluated at the time of data extraction, the safety of the novel food was confirmed in 79 cases, while 12 applications were rejected following evaluation. This corresponds to a positive opinion rate of 86.81%.
The main reasons for rejection were safety concerns (n = 8). Other reasons included the inability to identify the source of the novel food (n = 1) and poor application quality, including the lack of pertinent responses from the applicant (n = 3).
Duration of whole procedure timeline (W)
Overall, the average time from dossier submission to EFSA’s adoption of the opinion (AB + C) was 889 ± 433 days (range: 289–2255 days). The total time from submission to publication of EFSA opinion is (W) averaged 937 ± 436 days (range: 330–2314 days), which corresponds to approximately 31 ± 14 months, or 2.56 ± 1.19 years.
Figure 3 illustrates the timeline of NF applications in Europe, from submission to the publication of EFSA opinion.
Analysed timelines of NF applications in Europe, from submission to the publication of EFSA opinion. Numbers represent mean ± SD.
Applications rejected by EFSA due to non-compliance with the transparency regulation
Thirty applications were rejected by EFSA due to non-compliance with Article 32b of the Transparency Regulation, which mandates prior notification of studies. As this regulation came into force on March 27, 2021, only applications submitted after that date were affected (13 applications in 2021, 10 in 2022 and 7 in 2023). Among these, only two applications had entered the scientific evaluation phase when they were deemed not valid; the remaining 28 applications were still in the suitability check phase (i.e. intake) at the time of rejection.
The average time to rejection, defined as the period between EFSA’s receipt of the application and its rejection (E) was 297 ± 121 days (i.e. 10.6 ± 4.3 months), with a minimum of 74 days (2.6 month) and a maximum of 570 days (20.4 months). A slight decrease in the time needed to reject the application is noted (334 ± 90 days for applications submitted in 2021, 308 ± 132 days for those submitted in 2022, and 214 ± 130 days for those submitted in 2023).