Australia’s medicines regulator concedes its laws have failed to slow a growing number of blood toxicity cases attributed to a common over-the-counter vitamin.
The Therapeutic Goods Administration (TGA) also admitted some associated effects — including irreversible nerve damage — were a bigger problem than the agency first realised.
“The [legislation] changes from a few years ago, we’ve seen, were not sufficient to reduce the rate of the level of toxicity from B6,” the department’s chief medical adviser Robyn Langham told 7.30.
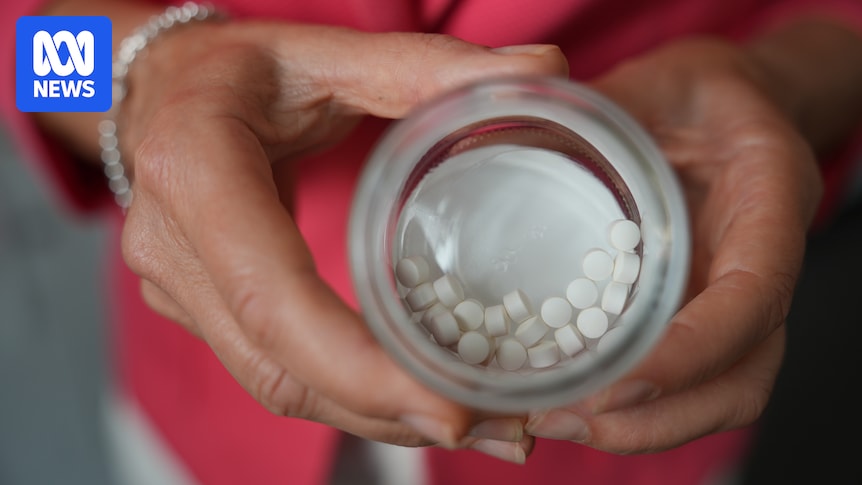
Many supplements containing B6 have levels well above the recommended daily amount. (ABC News: Jerry Rickard)
In its manufactured form, vitamin B6 — often labelled pyridoxine, pyridoxal, or pyridoxamine — is added to thousands of Australian products including foods, energy drinks, diet shakes and supplements.
Overconsumption of the synthetic vitamin can cause life-altering issues including peripheral neuropathy, a nerve damage complication causing tingling, numbness, burning or weakness in limbs.
“It’s just shocking,” said clinical pathologist David Kanowski, who runs a clinic testing B6 blood samples from across Australia.
Dr David Kanowski says he’s seen a “massive” increase in the number of people having B6 blood tests. (ABC News: Tom Hartley)
“Sometimes we talk to doctors who think it’s a clerical error because the toxicity results are so high, and we say, ‘No it’s real. You need to sort it out,'” he said.
Dr Kanowski has noted a “massive” increase in the number of people coming forward for B6 blood tests; the figure doubling since January to more than 10,000 each month.
Looking at the data for May, he said 4.5 per cent, or 450 people, returned results showing they were “very likely” to develop a neuropathy.
Dr David Kanowski believes B6 is sometimes added to supplements “as filler”. (ABC News: Tom Hartley)
A quarter of the samples, or 2,500 people, would “possibly, but not probably” also be showing symptoms.
“One big lot [of cases] was caused by magnesium supplements, and I think it was possibly added as filler,” Dr Kanoski said.
“I’m not sure the technical reasons why it was there.”
High levels of B6 in supplementsLoading…
The TGA is responsible for approving the ingredients used in complementary medicines in Australia.
B6 is registered in more than 1,500 over-the-counter supplements, from magnesium to multivitamins.
Eighty per cent of them contain levels well above the recommended daily amount of 1.7mg.
7.30 asked Blackmores Group, one of Australia’s biggest supplements manufacturers, why many of its products contain B6 in such high levels.
The company declined an interview but said all its products “including those containing vitamin B6, are developed in strict accordance with the regulatory requirements of the TGA”.
Loading…
While its own interim report has found “the benefits of supplemental intake of vitamin B6 to be negligible”, the TGA has joined the list of organisations that cannot or will not say why B6 is added to so many products.
“I’m not really able to answer why the commercial decisions are made by the different sponsors,” Professor Langham said.
She was quick to defend the TGA’s “regulatory frameworks” as “very robust”.
“They exist so that the safety of the Australian community is held right at the centre of everything that we do,” she said.
Professor Robyn Langham defended the TGA’s regulatory framework as “very robust”. (ABC News: Simon Winter)
“And when we see emerging issues such as this one, we are able … to develop tighter regulations.”
After months of pressure to act, the agency handed down several recommendations on Friday, based on the advice of a committee tasked to investigate the burgeoning B6 problem.
It was critical of the complementary sector, describing product labelling as “inconsistent and confusing”, and called for clearer information and the rescheduling of supplements containing more than 50 milligrams of the vitamin, which would move several products off shelves and behind counters.
“That will give the Australian community more protection and a greater sense of safety,” Professor Langham said.
Changes ‘too far away’
Chemist Caroline Diamantis is worried the TGA’s proposed B6 changes don’t go far enough. (ABC News: Craig Hansen)
The TGA last tightened the rules around B6 in 2022, requiring supplement companies to put warnings on products with more than 10mg.
But many sufferers and medical professionals say the changes did not go far enough.
And there are new concerns that the latest round of proposed changes will not prevent toxicity for people taking multiple supplements at once.
“It could still easily be an overdose over a period of time, if you’re taking three or four complementary medicines with lower dosages,” community pharmacist Caroline Diamantis told 7.30.
The complementary medicines sector argues that B6-related incidents are “extremely rare”, according to Complementary Medicines Australia boss John O’Doherty, “based on a comparison of sales data with the TGA’s public database”.
While that data shows more than 170 adverse events, the TGA’s Professor Langham said it could not be used to make that case.
“I think it’s sufficient to say that our database of adverse events doesn’t necessarily reflect the true incidence of what’s happening in the community,” she told 7.30.
“It’s also important to understand that because something is reported, it doesn’t necessarily mean that there’s a definite attribution of cause there.”
Caroline Diamantis says 2027 is “too far away” for the implementation of the proposed changes. (ABC News: Craig Hansen)
Ms Diamantis is also concerned the proposed implementation of the TGA’s regulatory changes, February 2027, “is too far away”.
“Personally I feel like that’s too long because there’s another year or more where people could still be experiencing side effects,” she said.
Professor Langham said the complexities of the changes meant the process was “a long one”.
“We’re going to have to look at reformulating their products, which will mean either transitioning them out of the market or reducing the dosage that’s in there,” she told 7.30.
“And there’s also another element of changes that the TGA is looking to make from a legal perspective to our legislation around some of the labelling and also around some of the lists of medicines, what we call our permissible ingredients list.
“It’s done so that things can be done carefully and properly, and so that Australians can feel confident, ultimately, that the correct decision is being made.”
The TGA will accept submissions on its “interim decision” until July 27, 2025, which will be considered by a senior medical officer before a final decision is made.
Watch 7.30, Mondays to Thursdays 7:30pm on ABC iview and ABC TV
Contact 7.30
Do you know more about this story? Get in touch with 7.30 here.