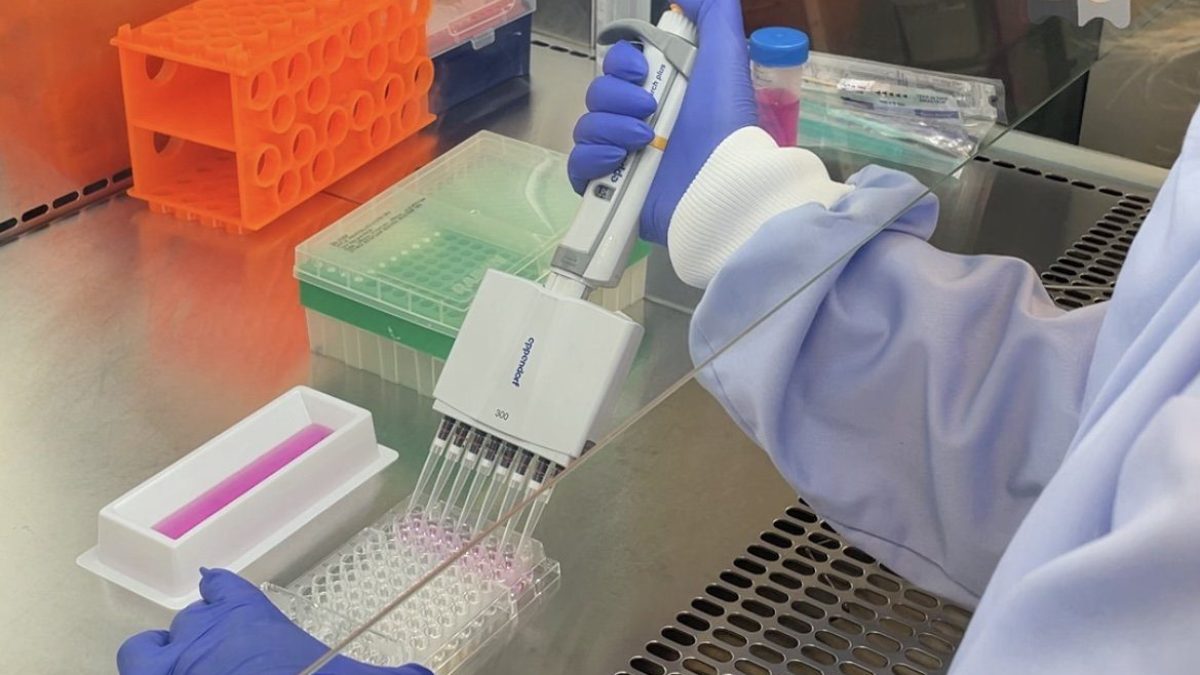
Doctors and scientists are hopeful that the non-antibiotic treatment could ultimately help millions of patients
Scientists are developing what they hope to be an alternative treatment for chronic urinary tract infections (UTI), which could help millions of patients suffering from the agonising condition.
Although tests are at an early stage, they hope the new drug will ultimately offer a much-needed alternative NHS treatment for patients who are unable to take antibiotics.
Chronic UTI, which affects an estimated 1.7 million women in Britain, according to the charity CUTIC, begins with an acute infection but becomes chronic when bacteria in the urine embed themselves into the bladder lining where immune cells cannot easily reach.
Some of the bacteria which cause UTIs have become resistant to routine antibiotics prescribed by GPs, meaning the condition is very difficult to treat and can cause ongoing, detrimental pain.
The current treatment for chronic UTI is to fight bacteria through traditional antibiotics – which is not suitable for all patients and risks contributing to antibiotic resistance.
As antimicrobial resistance becomes an increasing problem worldwide, it is more important than ever for scientists to find alternative treatment options.
The i Paper was granted exclusive access to one of the laboratories at the forefront of the search to find new treatment options.
Here is how the new treatment might work – and how soon it could be available to patients.
How would a non-antibiotic treatment work?
Scientists are working on a non-antibiotic, locally administered therapy that uses an antibacterial polymer.
This is a material commonly used to prevent bacteria from growing on surfaces, including in healthcare settings to avoid infections on devices such as catheters or in food packaging.
It is being developed by nanotechnology firm Tecrea, in partnership with The Bladder Infection and Immunity Group (BIIG), a research group at University College London (UCL) headed by leading chronic UTI doctors Rajvinder Khasriya and Harry Horsley.
 Leading chronic UTI doctor Rajvinder Khasriya, centre, with scientists Mafalda Castro, left, and Josephous Miculob (Photo: The i Paper)
Leading chronic UTI doctor Rajvinder Khasriya, centre, with scientists Mafalda Castro, left, and Josephous Miculob (Photo: The i Paper)
The research is taking place at the London BioScience Innovation Centre, hosted by the Royal Veterinary College (RVC), and was set up by Liam Good, a professor of microbiology and biotechnology at the RVC and co-founder of Tecrea.
The formulation is still being finalised, but it is likely to be delivered directly into the bladder via catheter, which would need to be instilled once per week.
Scientists Mafalda Castro and Josephous Miculob said that it is made up of two parts, an antimicrobial polymer called Nanocin and an analgesic drug, meaning pain relief.
“When you combine the two, you make something we call micelles, which have both the antimicrobial and pain-killing properties of both parts,” Castro said.
She continued: “When you make micelles using Nanocin, you can potentially improve the delivery and retention of the pain killer, improving its duration and effect.
“On the other hand, the Nanocin polymer targets the bacteria that cause UTI, even the ones that are hard to reach by traditional antibiotics or those that have developed antibiotic resistance. And it does this while potentially promoting a healthy microbiome.”
When will the drug be available to patients?
While doctors caution that further tests will be needed before putting the treatment forward for regulatory approval, if all goes well, there is the potential that this could be made available on the NHS in five years.
Dr Khasriya, an NHS urogynaecologist who runs the Lower Urinary Tract Symptoms (Luts) Service at the Whittington Hospital, said: “We want it to be used in patients in the NHS. The NHS wants to reduce antimicrobial prescribing, so absolutely, that would be the aim.”
Once the scientists have collected enough pre-trial data, they will begin clinical trials in the human bladder.
The treatment will then need to be approved as safe and effective by the Medicines and Healthcare products Regulatory Agency (MHRA).
 Castro at work in the lab (Photo: The i Paper)
Castro at work in the lab (Photo: The i Paper)
Then, the National Institute for Health and Care Excellence (NICE) will assess the new health technology and decide whether it offers value for money within the NHS.
Castro, who has suffered from recurrent UTIs herself, said she has had more than 10 infections in just one year, which is why she is “so passionate” about the project.
“I need this as an alternative because antibiotics didn’t solve my problem,” the principal scientist on the project said.
Miculob said that the goal of the project is to make a formulation that “targets the infection side of UTI, but also the anti-pain side of UTI”.
“Everyone goes for the infection because it is the root cause, but everyone seems to forget that UTIs – chronic UTIs especially – are such a burden in women, where they suffer through this pain daily,” he said. “So we thought it was really important to also address pain.”
 Scientists are hopeful that the new treatment will target the pain and infection (Photo: The i Paper)
Scientists are hopeful that the new treatment will target the pain and infection (Photo: The i Paper)
Dr Khasriya believes the new treatment has opened the door to finding a potential cure for the debilitating condition.
She said: “If we can get the right research done, get investment into Tecrea so that they can do the work, I don’t think a trial in humans – into the bladder – is very far off, maybe a few years. To then roll that out to other patients will probably take another few years.”
“If we had had a magic money tree, and all our dreams came true, probably something like five years. That is not a long time, if we think about it, because this product is already developed in a lot of ways.”
Doctors and scientists hope the treatment can be used for patients with recurrent UTIs, meaning they have more than three infections a year, and those with a chronic infection, meaning they have daily symptoms.
Dr Khasriya said she hopes the treatment will have a “huge impact” on patients who are currently suffering from daily “relentless” symptoms.