Nucleic Acid Therapeutics Market
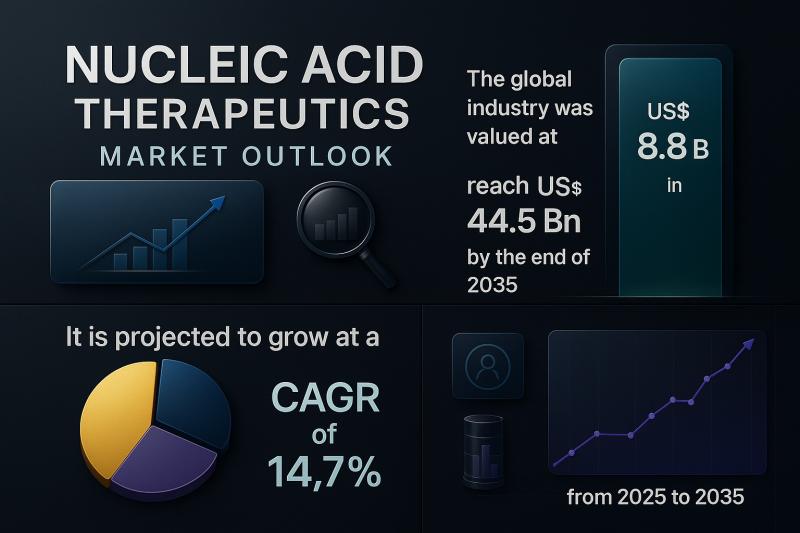
The landscape of modern medicine is undergoing a paradigm shift as nucleic acid-based treatments move from experimental science to clinical reality. These therapies, which leverage the power of RNA and DNA to treat and potentially cure disease at the genetic level, are no longer the promise of the future-they are rapidly becoming a foundational pillar of personalized medicine. According to recent projections, the global nucleic acid therapeutics market, valued at USD 8.8 billion in 2024, is set to soar to USD 44.5 billion by 2035, expanding at an impressive compound annual growth rate (CAGR) of 14.7%. This post offers a deep dive into the dynamics fueling this growth, the technologies shaping it, and the therapeutic breakthroughs that are redefining healthcare across genetic disorders, oncology, and beyond.
Access key findings and insights from our Report in this sample – https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=86333
Understanding Nucleic Acid Therapeutics: Precision Medicine at Its Core
Nucleic acid therapeutics encompass a broad spectrum of treatments that utilize genetic material-either DNA or RNA-as therapeutic agents. The field includes antisense oligonucleotides (ASOs), small interfering RNAs (siRNA), gene therapies, aptamers, and other modalities such as mRNA vaccines and therapies. Unlike conventional drugs that typically modulate proteins post-translationally, nucleic acid drugs intervene upstream in the disease process, targeting gene expression or correcting genetic mutations directly.
These therapies are being researched and deployed to combat rare genetic diseases, cancers, neuromuscular disorders, infectious diseases, and autoimmune conditions. The potential is immense: by intervening at the genetic level, these therapies offer unprecedented specificity, personalization, and the potential for long-term correction or even cure of diseases that were once considered intractable.
Key Market Drivers: Why the Future Belongs to Genetic Medicines
Rising Prevalence of Genetic Disorders and Unmet Clinical Needs
One of the central forces propelling the nucleic acid therapeutics market is the global rise in genetic and chronic diseases. Disorders like Duchenne muscular dystrophy (DMD), cystic fibrosis, sickle cell anemia, and thalassemia affect millions globally and often have limited treatment options. The National Organization for Rare Disorders reports that DMD alone occurs in about 1 in every 3,500 male births, driven by mutations in the DMD gene which disrupts dystrophin production. Nucleic acid therapies offer a direct solution to this problem, either by correcting the gene defect, introducing functional gene copies, or silencing harmful genetic expressions.
The increasing capabilities in genetic screening, prenatal diagnostics, and biomarker discovery are also contributing to the growth. These tools allow for early detection, often before symptoms appear, making preventive or preemptive treatment possible. As healthcare systems around the world improve their infrastructure and embrace personalized medicine, demand for nucleic acid therapeutics is expected to grow significantly.
Accelerated Regulatory Pathways and Government Support
The regulatory environment has evolved to accommodate and promote innovation in genetic medicine. Agencies like the U.S. FDA and EMA have developed expedited approval pathways to support treatments for rare and life-threatening diseases. Programs such as Fast Track, Breakthrough Therapy Designation, and Orphan Drug Designation are critical in reducing time-to-market for nucleic acid therapies.
These designations bring significant benefits, including rolling reviews, reduced application timelines, and increased engagement with regulatory bodies, which allows for more agile development strategies. Given the often-urgent nature of genetic diseases, such pathways are essential for bringing cutting-edge treatments to patients faster and more efficiently, acting as a catalyst for continued investment and innovation in the field.
Technological Innovations: A Pipeline Powered by Science
Antisense Oligonucleotides (ASOs): The Market’s Leading Segment
In 2024, ASOs emerged as the leading therapy type in the nucleic acid therapeutics market. These short, synthetic DNA or RNA fragments are designed to bind to specific mRNA molecules to alter gene expression. Their mechanism allows them to either block the production of a protein or correct a genetic mutation at the RNA level, making them highly versatile tools in the treatment of rare genetic diseases and cancers.
ASOs have seen increasing clinical validation, with several FDA-approved therapies already on the market and many more in clinical trials. Companies are actively working to enhance the stability, specificity, and delivery mechanisms of ASOs to maximize their therapeutic potential. As these challenges are addressed, ASOs are expected to expand into broader disease categories, further boosting their market dominance.
Gene Therapy and RNA Interference (RNAi): Long-Term Solutions
Gene therapies represent another key growth area, offering the possibility of curative treatment by inserting, deleting, or editing defective genes. Advances in CRISPR, AAV vectors, and non-viral delivery systems have expanded the range of treatable conditions and improved safety profiles.
RNA interference (RNAi), particularly siRNA therapies, are gaining traction for their ability to selectively silence disease-causing genes. Companies like Alnylam Pharmaceuticals have pioneered this space with approved therapies for rare genetic diseases, and their success has attracted significant interest and investment into RNAi platforms.
Delivery Methods: Overcoming the Final Hurdle
A critical challenge in nucleic acid drug development is the efficient delivery of therapies into target cells or tissues. The market is segmented into viral vector-based systems (such as AAV and lentivirus) and non-viral delivery systems (such as lipid nanoparticles and polymer-based carriers). The COVID-19 pandemic demonstrated the power of lipid nanoparticle technology in mRNA vaccine delivery, accelerating research and confidence in non-viral systems.
Looking ahead, the focus will be on improving targeted delivery, reducing immunogenicity, and enhancing cell-specific uptake. As delivery technologies mature, they will unlock broader applications for nucleic acid therapeutics in oncology, cardiovascular disease, and even CNS disorders.
Explore our report to uncover in-depth insights – https://www.transparencymarketresearch.com/nucleic-acid-therapeutics-market.html
Regional Outlook: North America at the Helm of Genetic Innovation
North America, led by the United States, remains the dominant force in the nucleic acid therapeutics market. The region boasts advanced healthcare infrastructure, a strong network of biotech startups and pharmaceutical giants, and a robust regulatory framework that supports innovation. The U.S. FDA’s proactive role in fast-tracking nucleic acid therapies, coupled with public-private collaborations, has enabled rapid clinical development and commercialization.
Meanwhile, Europe and Asia Pacific are emerging as key contributors. Countries like Germany, the U.K., Japan, and China are ramping up investments in precision medicine, genomics, and local biopharmaceutical manufacturing. In particular, Asia Pacific offers cost-effective clinical trials and a large patient pool, making it a strategic hub for future expansion.
Competitive Landscape: Mergers, Milestones, and Market Movers
The nucleic acid therapeutics market is characterized by intense competition and strategic alliances among major pharmaceutical players. Leading companies such as Novartis, Pfizer, Sanofi, Novo Nordisk, AstraZeneca, Amgen, and Sarepta Therapeutics are investing in both in-house development and external partnerships to expand their pipelines.
Want to know more? Get in touch now. -https://www.transparencymarketresearch.com/contact-us.html
Recent notable developments include:
Nov 2024: Novartis acquired Kate Therapeutics, a preclinical firm focused on AAV-based gene therapies for neuromuscular diseases, for USD 400 million, with milestone payments potentially reaching USD 1.1 billion.
Nov 2024: Sarepta Therapeutics entered into a global licensing deal with Arrowhead Pharmaceuticals, securing access to siRNA programs for muscle and CNS disorders, with an upfront payment of USD 500 million and USD 325 million in equity investments.
These deals underscore the high strategic value of nucleic acid therapies in future healthcare portfolios and reflect a race to secure first-mover advantages in next-generation treatments.
Explore Latest Research Reports by Transparency Market Research:
Hematocrit Test Devices Market – https://www.transparencymarketresearch.com/hematocrit-test-market.html
Cellulite Treatment Market – https://www.transparencymarketresearch.com/cellulite-treatment-market.html
Implantable Cardiac Monitor Market – https://www.transparencymarketresearch.com/implantable-cardiac-monitors-market.html
Surgery Tables Market – https://www.transparencymarketresearch.com/surgery-tables-market.html
Microdermabrasion Devices Market – https://www.transparencymarketresearch.com/microdermabrasion-devices-market.html
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
Email: sales@transparencymarketresearch.com
Follow Us: LinkedIn| Twitter| Blog | YouTube
This release was published on openPR.