Scientists have used a single injection to correct gene mutations caused by an ultra-rare disease, improving symptoms and survival rates in mice.
Published in Cell, the gene editing study targeted the 2 most common mutations that cause alternating hemiplegia in childhood (AHC).
AHC is a rare neurological disorder affecting 1 in a million people. Symptoms, which usually begin before the age of 18 months, include weakness and paralysis in one or both sides of the body, muscle stiffness and, in some cases, seizures.
Current treatments help with symptom management but there is no known cure for AHC.
Markus Terrey, neuroscientist at JAX, co-led the pioneering study that corrected AHC-causing mutations directly in the brain of mice. Credit: The Jackson Laboratory.
The researchers consisted of a team from the Rare Disease Translational Centre, the Broad Institute and the not-for-profit, RARE Hope.
Mice models were previously developed by Markus Terrey and Cathleen Lutz, vice president of the Rare Disease Translational Centre.
“Five years ago, people would have thought that going into the brain of a living organism and correcting DNA was science fiction. Today, we know this is doable,” says Terrey, who co-led the study.
“Doing this directly in the brain of a living organism is scientifically fascinating. You can go into the brain, fix the mutation, and have the cells corrected for the rest of their life.”
The team used prime editing to correct 85% of the faulty gene mutations in the brain.
“This level of editing efficiency in the brain is really quite remarkable,” says Lutz.
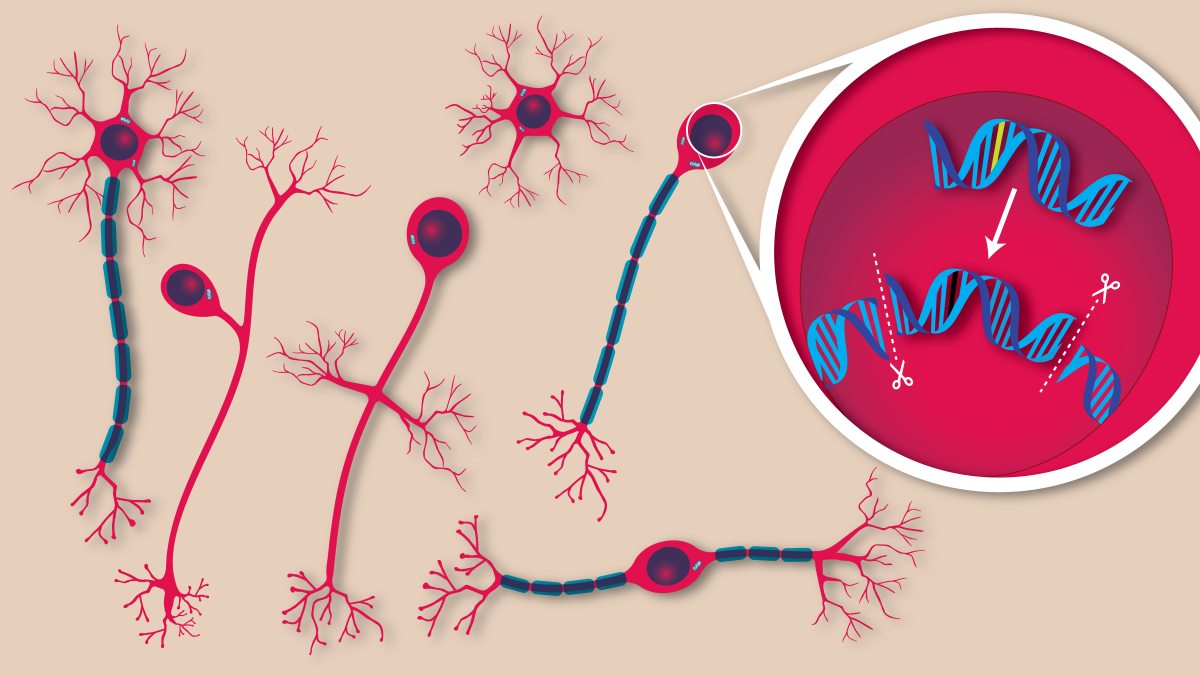
Illustration of gene editing in neurons. Credit: The Jackson Laboratory.
Prime editing uses CRISPR technologies and reverse transcriptase enzymes to create new single strands of DNA that can be inserted into existing DNA sequences.
The correction restored normal protein function allowing for a reduction in seizures and motor skill improvement. It also extended the lifespan in mice.
“Up until this point, we didn’t know if this was a disease that could be rescued postnatally,” says Nina Frost, a co-author of the study and mother of a daughter with AHC. “To see data that showed not just molecular correction in cells, but a functional rescue in mouse behavior, was an incredibly exciting moment.”
Cathleen (Cat) Lutz, vice president of the Rare Disease Translational Center at JAX, emphasized the broader implications of the breakthrough, calling it a major step toward personalized therapies for rare genetic disorders. Credit: The Jackson Laboratory.
This discovery comes off the back of previous work by the same team which used gene editing therapy to treat an infant with a rare genetic liver disorder at the Children’s Hospital of Philadelphia.
The team is now shifting its focus to finding the safest and most effective time to edit the gene to reverse AHC in mice.
“If we can do it for one gene variant – and we already have 5 in the paper – we can reasonably assume that we can do this for other variants as well,” says Terrey. “We can expand this work towards other rare diseases, because 80% of them are genetic. We know exactly where the problem is.”
They hope that the success of this study will offer new approaches to how neurological diseases are treated and potentially prevented.
“This study is an important milestone for prime editing and one of the most exciting examples of therapeutic gene editing to come from our team,” says David Liu, a co-senior author of the study who helped develop prime editing in 2019.
“It opens the door to one day repairing the underlying genetic causes of many neurological disorders that have long been considered untreatable.”
Current estimates suggest there are between 5,000 and 8,000 diseases that arise from mutations on a single gene.
“While the incidence of this disease is very rare, the incidence of monogenic, rare conditions that could be addressed with gene editing is actually a really big number. The impact of this success resonates far beyond AHC,” adds Frost.