Nucleic acid-based therapeutics such as mRNA, small interfering ribonucleic acid (siRNA) and antisense oligonucleotides (ASOs) represent a groundbreaking approach in medicine, leveraging the power of RNA and DNA to treat – and sometimes cure – a wide range of diseases at the molecular level.
Unlike traditional treatments that often focus on managing symptoms, these therapies address underlying genetic issues, offering more precise and potentially more durable solutions. By designing treatments based on an individual’s genetic profile, they can be highly personalized, leading to the possibility of improved outcomes for patients with rare genetic disorders, cancers, viral infections and other conditions.
Perhaps the most widely publicized and well-known application of nucleic acid delivery has been the COVID-19 vaccines. mRNA vaccines like Pfizer-BioNTech’s BNT162b2 (Comirnaty) and Moderna’s mRNA-1273 (Spikevax) represented a groundbreaking approach to immunization, utilizing mRNA to instruct cells to produce a harmless piece of the virus’s spike protein, prompting an immune response against the virus.
Several siRNA and ASO-based therapies have also been approved, including treatments for hereditary transthyretin amyloidosis (Patisiran), acute hepatic porphyria (Givosiran), Duchenne muscular dystrophy in patients with a specific mutation (Eteplirsen) and spinal muscular atrophy (Nusinersen).
While these vaccines and therapeutics offer important health benefits, delivering these therapies to their intended sites of action in the body has been a formidable challenge due to many factors:
- Stability and degradation: Nucleic acids are unstable and susceptible to degradation by nucleases in biological environments, which can lead to reduced efficacy.
- Cellular uptake: The nucleic acid must move through the cell membrane, which is not readily permeable to large, negatively charged nucleic acid molecules.
- Targeted specificity: Precision delivery to the intended cells or tissues is essential for minimizing off-target effects. Without targeted delivery, nucleic acid therapeutics may affect unintended cells, potentially leading to adverse effects.
- Endosomal escape: Once inside the cell, nucleic acids often end up in endosomes, which result in their degradation.
- Immunogenicity: Some nucleic acid formulations can trigger immune responses, leading to inflammation or reduced therapeutic efficacy.
- Dosing and formulation: Determining the appropriate dosage and formulation for nucleic acid therapeutics can be complex and require the ability to address solubility, stability and release kinetics.
- Manufacturing and scale-up: Producing these therapeutics at scale while ensuring quality and consistency can be challenging, particularly with the need for complex delivery systems and formulations.
Addressing these challenges is crucial for successfully developing and commercializing a broader array of nucleic acid therapeutics, which hold great promise for treating such a wide variety of conditions and diseases.
Developing a new approach to delivery
Designing an effective delivery system that ensures the nucleic acid reaches target cells or tissues without being degraded or causing off-target effects is one of the most critical challenges facing the field of gene therapy. A therapy that can’t reach its intended target will not succeed, as it could either fail to elicit the desired biological response or, worse, cause unintended consequences in healthy cells. Success in this area is essential to unlock the full potential of gene therapy for treating a wide range of genetic disorders.
Historically, delivery systems have included viral vectors and non-viral vectors, each with its own set of limitations.
Adeno-associated virus (AAV) vectors are widely used in gene therapies due to their ability to efficiently deliver genetic material into cells. They have relatively low immunogenicity, making them safer for repeated dosing compared to other viral vectors, and they provide long-term gene expression in non-dividing cells.
AAV vectors do have limitations, however. Their small packaging capacity restricts the size of the genetic material they can carry, which poses a challenge for diseases requiring larger genes. Additionally, pre-existing immunity to AAV can reduce the effectiveness of treatment, and the risk of unintended integration into the host genome, though rare, may potentially cause harmful mutations. This approach also presents challenges related to chemistry, manufacturing and controls (CMC), as well as batch-to-batch consistency.
Lentivirus vectors are also used for delivering gene therapies as they can integrate genetic material into the host cell’s genome, ensuring long-term and stable expression. This feature makes them ideal for treating chronic conditions, as the therapeutic gene remains active even as cells divide, making lentivirus vectors especially useful in targeting rapidly dividing cells, such as those in the bone marrow. Lentiviruses have a relatively large packaging capacity, allowing them to carry larger genes compared to other viral vectors like AAV.
Integration of lentiviral DNA into the host genome does pose a risk of insertional mutagenesis, however, which creates the risk of cancer or other genetic disruptions. Additionally, while lentivirus vectors have been engineered to be safer and non-replicative, there are still concerns about the potential for immune responses or viral recombination.
Non-viral vectors such as lipid nanoparticles (LNPs) have emerged as a powerful tool for delivery due to their ability to encapsulate and protect nucleic acids, facilitate cellular entry and exhibit relatively low immunogenicity. LNPs are used in the COVID-19 mRNA vaccines and for siRNA delivery.
There are also numerous clinical trials with LNPs that include various payloads. However, the majority of these are for liver-focused disease areas simply because that is where the delivery systems accumulate in the body when administered systemically. This limits the actual potential of genomic medicines and has led the field to expand the LNP delivery toolbox beyond the liver. Many groups are currently trying to identify what characteristics are needed for these LNPs to allow delivery of the nucleic acid payload to specific cell types.
Building advanced LNP delivery systems
At Liberate Bio, we are advancing novel nanoparticle delivery vehicles for nucleic acid cargoes such as ASOs, siRNAs, mRNAs and encoded gene editors. The goal is twofold: first, to partner with other companies that need them for their respective technologies; and second, to build a proprietary pipeline where they are used for various modalities, all nucleic acid-based, for different therapeutic areas of interest.
To achieve tissue-specific targeting, we have established a high-throughput, in vivo screening platform that allows us to evaluate a wide range of nanoparticle materials, chemistries and formulations to identify those with distinct tissue-targeting capabilities. The particles can differ by way of size, shape, overall structure and charge, or changes to the nanoparticle that direct it to specific cell types.
Screening in physiologically relevant models, along with machine learning to identify novel lipids and LNP formulations, guides the design and optimization of new nanoparticle materials and formulations, enhancing their ability to selectively deliver therapeutics to specific tissues.
With this approach, the need to align different types of nucleic acid cargo and the delivery system is also addressed; for example, circular RNAs are fundamentally different cargoes, and self-amplifying RNAs are much larger. Importantly, the high-throughput screening capabilities have the power to first identify fundamentally new components for successful extrahepatic tissue-specific delivery, and then further improve those at a significantly faster pace than conventional delivery platforms.
A core component of the platform is protein barcoding and next-gen protein sequencing (NGPS) using the Platinum® instrument from Quantum-Si to screen LNP delivery vehicles in vivo. Protein barcoding makes the pre-clinical drug development process more efficient and cost-effective through multiplexed protein screening, to accelerate the path to first-in-human trials.
The protein barcoding process is very straightforward (Figure 1). Short sequences that encode unique protein barcodes are attached to the mRNAs being packaged into a range of LNPs with different chemistries and formulations. These LNPs are then administered to non-human primates. The LNPs are taken up by various tissues, and the mRNAs they carry are translated into proteins.
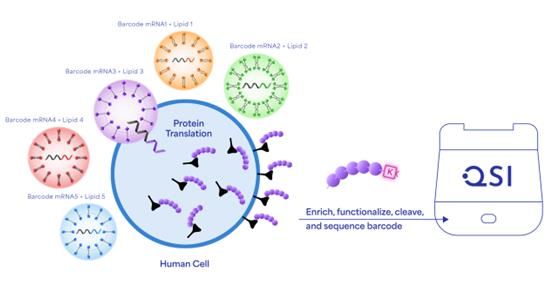
Figure 1: Screening nucleic acid delivery methods using protein barcodes. Credit: Quantum-Si.
With the use of this technique, each protein features a distinct barcode that corresponds to the specific LNP that carried it. By extracting proteins from different tissues and using NGPS to identify the barcodes, the identity of which LNP traveled to which tissue is revealed. This process allows the creation of extensive datasets and provides a comprehensive, unbiased understanding of how different LNP chemistries affect delivery and expression in a biologically relevant model.
Not only does integration of the barcoding application into the workflow accelerate discovery and screening efforts, but it also reduces costs and shortens timelines.
The real power of protein barcoding is the ability to evaluate multiple factors simultaneously, significantly accelerating the drug discovery process. Instead of the typical three- to five-year timeline, hits and leads can be identified much more quickly. This allows redirection of financial and human resources toward developing the actual gene therapies, rather than investing extensive time in early discovery work.
The future of nucleic acid therapeutics
The potential for nucleic acid-based therapies to revolutionize medicine is clear. By addressing the root causes of diseases at the genetic level, these therapies offer solutions for conditions that were previously considered untreatable. The challenge now is to continue improving the delivery systems that can unlock this potential. Whether through viral vectors, advanced LNPs or entirely new technologies, the future of nucleic acid therapeutics will depend on overcoming the challenges of stability, targeted delivery and manufacturing scalability.
The power of nucleic acid therapeutics is undeniable, and with continued research, we stand ready to transform how we treat a wide range of diseases – from rare genetic disorders to cancer and beyond. The road ahead is challenging, but the promise of these therapies to change lives makes it an exciting time for the field of genetic medicine.
